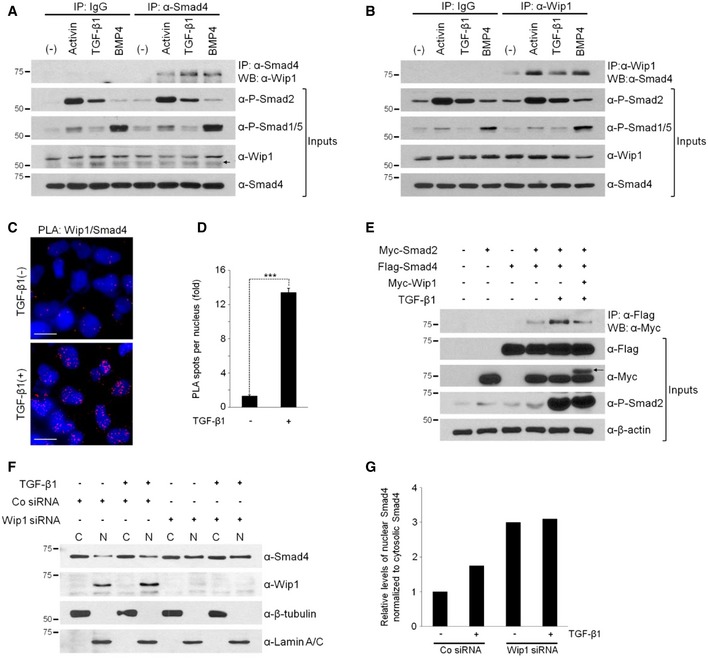
Figure 5. Wip1 interacts with Smad4 in vivo .
-
A, BWip1 and Smad4 form an endogenous protein complex. Cell lysates of HEK293T cells, which were stimulated or not with activin (10 ng/ml), TGF‐β1 (40 ng/ml), or BMP4 (20 ng/ml) for 1 h, were immunoprecipitated with anti‐Smad4 antibody followed by Western blotting with anti‐Wip1 antibody (A) and vice versa (B). (–), no treatment with protein ligand. IgG, control IgG. An arrow in (A) indicates non‐specific bands.
-
CUpon ligand stimulation, Wip1 forms a complex with Smad4 in the nucleus. HeLa cells were treated or not with TGF‐β1 (40 ng/ml) for 2 h, fixed, and then subjected to proximity ligation assays with primary antibodies against Wip1 and Smad4. Blue: DAPI; red: PLA signal. Scale bar, 20 μm.
-
DQuantitative analysis of Wip1/Smad4 interactions shown in (C). Data are represented as the mean ± SEM (n = 3 biological replicates). Twenty cells were analyzed per sample. ***P < 0.001 by Student's t‐test.
-
EWip1 interferes with the ability of Smad4 to bind to Smad2. HEK293T cells co‐transfected with the indicated constructs were treated or not with TGF‐β1 for 1 h and then harvested. An arrow denotes Myc‐tagged Wip1 protein.
-
FWip1 has a negative effect on nuclear accumulation of Smad4. HEK293T cells were transfected with Co siRNA or Wip1 siRNA, treated with TGF‐β1 (40 ng/ml, 1 h) 48 h after siRNA transfection and subjected to nuclear/cytoplasmic fractionation and Western blotting analysis. β‐tubulin and lamin A/C serve as cytoplasmic and nuclear markers, respectively. C, cytoplasm; N, nucleus. The experiment was repeated two times with similar results.
-
GQuantification of the levels of nuclear Smad4 normalized to those of cytoplasmic Smad4 shown in (F).
Source data are available online for this figure.
