Figure 1. The four families of mitochondrial metabolite transporters.
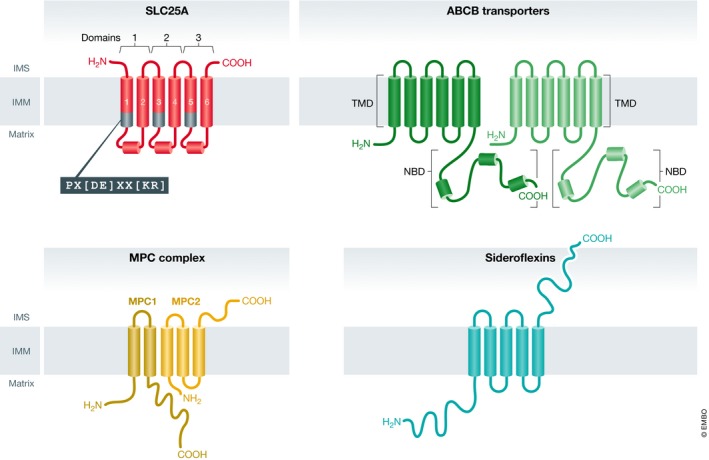
Illustrations of the four classes of mitochondrial metabolite carriers: SLC25A, ABCB transporters, mitochondrial pyruvate carrier (MPC) complex, and the sideroflexin proteins. Each SLC25A member is a pseudo‐symmetrical threefold structure with each domain containing two alpha helices connected by a soluble loop‐helix‐loop. The end of each odd‐numbered helix contains a signature carrier motif: PX[DE]XX[KR]. ABCB transporters of the mitochondria contain twelve membrane‐spanning helices regulated by nucleotide binding domains (NBDs). The MPC complex consists of two proteins: MPC1, a two‐pass transmembrane protein, and MPC2, believed to be a three‐pass transmembrane protein. These two individual units interact as a functional heterodimer. Sideroflexins are five‐pass transmembrane proteins.
