Abstract
Introduction
Helicobacter pylori (HP) infection is associated with many gastrointestinal disorders, including gastric cancer, and consensus guidelines recommend eradication after detection. There is a theoretical, yet uninvestigated, concern that HP treatment could increasing the risk of Clostridium difficile infection (CDI). Using data from a large cohort of patients with HP, we investigated whether HP eradication is associated with CDI.
Methods
Retrospective cohort study within the Veterans Health Administration on 38,535 patients (median age 61.8 years; 91.8% male) with detected HP between 1/1/1994–12/31/2018. Primary outcome was a positive laboratory test for CDI within 3 months of HP detection. Multivariable logistic regression evaluated: patient demographics, prior CDI, recent hospitalization, and whether the patient received HP eradication therapy (by antibiotic and regimen, including proton pump therapy). Secondary analysis of those treated evaluated whether eradication of HP was associated with CDI.
Results
Among 38,535 patients, 28,818 (74.8%) were treated for HP, and 284 (0.74%) developed CDI. In multivariable analysis, prominent factors included hospital discharge within 12 weeks (OR 2.15; 95% CI:1.22–3.77) and 4 weeks (OR 3.46; 95% CI:2.18–5.48), p<0.001, and prior CDI (OR 12.5; 95% CI:9.21–17.0, p<0.001). Treatment of HP was not associated with future CDI. In secondary analysis of those treated, confirmation of eradication was not associated with future CDI (OR 1.49; 95% CI:0.67–3.29).
Conclusions
In a large study of US patients with HP, we demonstrate that neither treatment nor eradication of HP is associated with CDI. Prior CDI and recent hospital discharge, known risk factors, are strongly associated. These findings suggest that treatment should be continued to prescribed when HP is detected.
INTRODUCTION
Infection with Helicobacter pylori (HP) is common, with an estimated 50% of the world affected, and can lead to a variety of gastrointestinal pathology, from ulcer disease to malignancy.1–3 As such, consensus guidelines recommend treating all known HP infections and subsequently testing for confirmation of eradication.4–6 Treatment consists of a multi-drug regimen for 1–2 weeks, and can include multiple antibiotics. Given the increasing awareness regarding untoward effects of antibiotics, such as increasing rates of resistance and side effects, there are those that caution against HP eradication.7–13 They argue that eradication of HP can be associated with antibiotic resistance, antibiotic associated diseases, and changes in the microbiome that could have downstream consequences. A particularly concerning, yet uninvestigated, area is the association of HP treatment with Clostridium difficile infection (CDI).
CDI is one of the most common health care-associated infections, and results in significant morbidity and mortality in the US population.14, 15 Antibiotic stewardship has been shown to decrease the impact of CDI, and is an important public health initiative.16 Accordingly, the potentially deleterious effects of HP must be carefully balanced with untoward effects of antibiotics, including CDI. Previous literature regarding the development of CDI after HP eradication therapy has generally been limited to case reports, limiting the ability to draw conclusions.17–19 To our knowledge, no study has comprehensively studied CDI after HP, accounting for patient factors, risk factors for CDI (previous infection and recent hospitalization), antibiotic therapy, and presence of confirmed eradication. Here, we utilize the largest North American cohort of patients with HP to identify risk factors for future CDI, and the impact of HP treatment on future CDI.
METHODS
This retrospective cohort study was conducted within the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW), which includes data from the unified electronic medical record of all VHA facilities (i.e., hospitals and outpatient).
Study cohort
We identified patients with HP infection, and this cohort has been extensively described elsewhere.20 Briefly, patients with HP infection 1/1/1994 – 12/31/2018 were included based on: 1) endoscopic pathology by natural language processing, 2) positive stool antigen test, or 3) positive urea breath test. For patients with multiple criteria, the criterion with the earliest date was used. (Unique identifiers assured no duplications.)
Study outcomes
The primary outcome was a positive laboratory test for Clostridium difficile toxin or PCR within the Veterans Health Administration within the 3 months after HP detection.
Statistical analysis
Logistic regression was performed, and a multivariable analysis was conducted evaluating the following co-variates: age, ethnicity, race, gender, smoking history, 21 poverty level of zip code where patient resided at HP diagnosis, history of prior CDI, recent hospitalization (within 4 weeks or 12 weeks),22, 23 and whether the patient received prescription for an eradication regimen for HP (subcategorized by regimen received). Zip code-level poverty was based on 2010 census data, categorized based on percentage of people within a zip code below the federal poverty line. Receipt of HP treatment was defined as receiving a recommended antibiotic regimen after HP diagnosis at the VHA using prescription filling data at any inpatient or outpatient VHA facility.20 Exploratory analyses were conducted evaluating receipt of HP treatment (yes/no), by antibiotic class, and by antibiotic regimens. Similarly, for proton pump inhibitor (PPI) therapy, we evaluated PPI choice, dose (high-dose vs not, as per the United Kingdom National Institute for Health and Care Excellence classifications), and duration, whether the patient had been prescribed a PPI outside of the HP eradication period.
A secondary analysis evaluated whether confirmation of HP eradication amongst those who received prescription therapy for their HP diagnosis was associated with CDI, given varying rates of re-testing and successful HP treatment, as well as the potential for HP eradication to change the microbiome, leading to subsequent CDI.3, 4, 24 This analysis was restricted to those who received treatment for HP. Eradication was based on having either a negative stool antigen, urea breath test, and/or pathology (gastric biopsy on endoscopy) upon repeat testing. Failed eradication was defined as a positive stool antigen, urea breath test, and/or pathology, or a positive HP test after a prior negative test given that true re-infection is exceedingly rare. Patients without any eradication testing were considered as ‘unknown’ eradication status. HP status on pathology was determined by repeat natural language processing, which has been described elsewhere.20
Stata/IC 15.1 (College Station, TX) was used to perform backward selection, with inclusion of all clinically significant sub-hazard ratios (SHRs), where P<0.10. The Institutional Review Boards of the Corporal Michael J. Crescenz VA Medical Center and the University of Pennsylvania approved this study.
RESULTS
We identified 38,535 patients with detected HP infection on endoscopic pathology, urea breath testing, or stool antigen. Of these 38,535 patients, 284 (0.74%) had subsequent CDI. Among the 38,535, 28,818 (74.8%) were treated for HP. Table 1 compares those patients who developed CDI versus those who did not. Patients who developed CDI were older (median age 64 vs 62 years, p=0.001) and more likely to be of non-Hispanic or Latino ethnicity (70.1% vs 66.3%, p=0.01) without significant differences in gender, race, smoking history, or poverty level. They were more likely to have a previous history of CDI (58.1% vs 4.1%, p<0.001) and a recent hospitalization, either within 4 weeks (15.5% vs 0.5%) or 12 weeks (17.3% vs 0.7%) of CDI, p<0.001. There was no significant difference in method of HP diagnosis. Those who developed CDI were less likely to have received treatment for HP within the VHA (66.2% vs 74.8%, p<0.001). When evaluating antibiotics, those who developed CDI were less likely to have been prescribed a regimen that included amoxicillin (44.4% vs 56.8%, p<0.001) or clarithromycin (45.4% vs 57.1%, p<0.001), without significant difference in metronidazole prescription (20.1% vs 17.4%, p=0.23) or levofloxacin (1.4% vs 1.2%. p=0.70). There was no difference between the PPI used during HP eradication among those who go on to develop CDI and those who do not. Patients who developed CDI were not significantly more likely to have been prescribed a high dose PPI (11.4% vs 10.7%, p=0.77) or been prescribed PPI therapy outside the HP eradication period (9.6% vs 11.7%, p=0.57).
Table 1.
Patients who developed Clostridium difficile infection (CDI) versus those who did not
| Did not develop future CDI (n = 38,251) | Developed future CDI (n = 284) | p-value | |
|---|---|---|---|
| Age at H pylori diagnosis, years (median, IQR) | 62 (53, 69) | 64 (56.5, 70.5) | 0.001 |
| Male gender | 35135 (91.9%) | 253 (89.1%) | 0.090 |
| Race | 0.30 | ||
| White | 16,210 (42.4%) | 134 (47.2%) | |
| Black or African American | 11,523 (30.1%) | 75 (26.4%) | |
| American Indian/Alaska Native | 303 (0.8%) | 1 (0.4%) | |
| Asian | 221 (0.6%) | 1 (0.4%) | |
| Native Hawaiian/Pacific Islander | 305 (0.8%) | 0 (0.0%) | |
| Unknown | 9,689 (25.3%) | 73 (25.7%) | |
| Ethnicity | 0.01 | ||
| Not Hispanic / Latino | 25,346 (66.3%) | 199 (70.1%) | |
| Hispanic or Latino | 4,318 (11.3%) | 16 (5.6%) | |
| Unknown | 8,587 (22.4%) | 69 (24.3%) | |
| Smoking history | 9,720 (25.4%) | 85 (29.9%) | 0.08 |
| Poverty level of zip code where patient resided at H pylori diagnosis | 0.62 | ||
| < 10% residing below poverty level | 7,849 (20.5%) | 65 (22.9%) | |
| 10 – 24.9% residing below poverty level | 17,981 (47.0%) | 129 (45.4%) | |
| 25 – 49.9% residing below poverty level | 9,629 (25.2%) | 70 (24.6%) | |
| ≥50% residing below poverty level | 1,100 (2.9%) | 5 (1.8%) | |
| Unknown | 1,692 (4.4%) | 15 (5.3%) | |
| Prior history of CDI | 1,583 (4.1%) | 165 (58.1%) | <0.001 |
| Recent hospitalization | <0.001 | ||
| None | 37,799 (98.8%) | 191 (67.3%) | |
| Within 12 weeks | 192 (0.5%) | 44 (15.5%) | |
| Within 4 weeks | 260 (0.7%) | 49 (17.3%) | |
| Method of H pylori diagnosis | 0.23 | ||
| Pathology | 26,688 (69.8%) | 185 (65.1%) | |
| Stool Antigen | 11,166 (29.2%) | 96 (33.8%) | |
| Urea Breath Test | 397 (1.0%) | 3 (1.1%) | |
| Received treatment for H pylori diagnosis | 28630 (74.8%) | 188 (66.2%) | <0.001 |
| H pylori regimen included: | |||
| Amoxicillin | 21,727 (56.8%) | 126 (44.4%) | <0.001 |
| Clarithromycin | 21,845 (57.1%) | 129 (45.4%) | <0.001 |
| Levofloxacin | 444 (1.2%) | 4 (1.4%) | 0.70 |
| Metronidazole | 6,640 (17.4%) | 57 (20.1%) | 0.23 |
| Rifabutin | 7 (<1%) | 0 (0.0%) | 0.82 |
| Tetracycline | 19 (<1%) | 0 (0.0%) | 0.71 |
| PPI that H pylori regimen included: | 0.28 | ||
| Omeprazole | 22,071 (81.5%) | 149 (84.7%) | |
| Pantoprazole | 3,094 (11.4%) | 15 (8.5%) | |
| Rabeprazole | 908 (3.4%) | 9 (5.1%) | |
| Dexlansoprazole | 2 (<1%) | 0 (0.0%) | |
| Lansoprazole | 1,011 (3.7%) | 3 (1.7%) | |
| High dose PPI use | 2,895 (10.7%) | 20 (11.4%) | 0.77 |
| PPI use outside of H pylori regimen | 0.57 | ||
| No | 23,725 (82.9%) | 158 (84.0%) | |
| Yes | 3,360 (11.7%) | 18 (9.6%) | |
| Unknown | 1,545 (5.4%) | 12 (6.4%) |
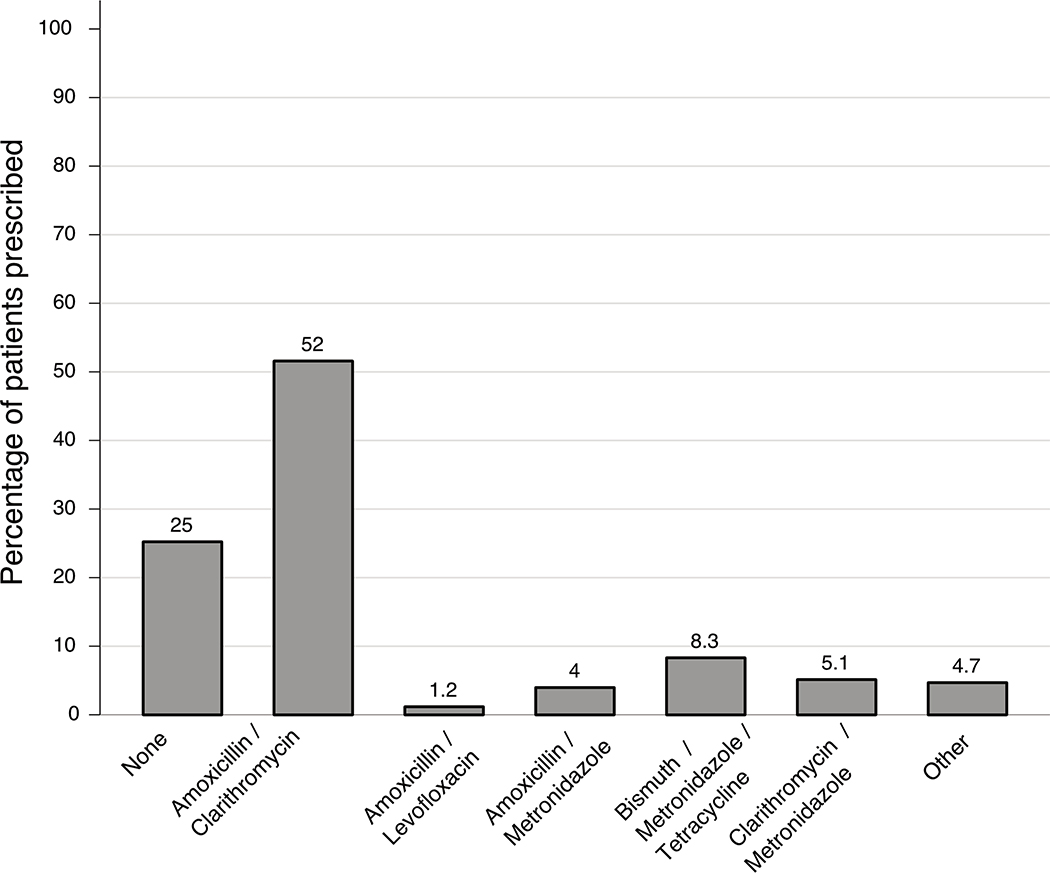
Figure 1 demonstrates the most commonly prescribed regimens (if any) for HP eradication. The most frequently regimen included amoxicillin and clarithromycin-based triple therapy in 19,871 (51.6%), followed by bismuth-based quadruple therapy in 3,196 (8.3%). Of the 38,535, 9,717 (25.2%) had no eradication regimen prescribed in the VHA.
Figure 1.
Bar graph displaying frequency of prescribed HP regimens (not including proton pump inhibitor prescription). Other includes rifabutin and other regimens as per the American College of Gastroenterology
In multivariable analysis, there were multiple factors associated with increased future risk of CDI (Table 2). The two most prominent included recent hospital discharge and prior CDI infection: recent hospital discharge within both 12 weeks (OR 2.15; 95% CI: 1.22–3.77) and 4 weeks (OR 3.46; 95% CI: 2.18–5.48), were associated with future CDI, p<0.001. A prior history of CDI was strongly associated with future CDI (OR 12.5; 95% CI: 9.21 −17.0). Treatment of HP was not associated with increased future CDI in multivariable analysis, either as a whole or when analyzed by HP eradication regimen or which antibiotic the patient was prescribed. Neither long-term PPI use or PPI use during HP eradication (evaluated by PPI choice, dose, and duration, with and without specific antibiotic regimen) was significantly associated with CDI. Patient demographics demonstrated that females were more likely to have future CDI (OR 1.74; 95%CI: 1.20–2.52, p=0.003) and those of White race were more likely to develop future CDI (see Table).
Table 2.
Risk factors for development of Clostridium difficile infection after detection of H pylori using multivariable logistic regression model
| Odds ratio (95% CI) | P-value | |
|---|---|---|
| Age at H pylori diagnosis * | 0.91 (0.87–0.96) | P<0.001 |
| Female gender | 1.74 (1.20–2.52) | 0.003 |
| Race | 0.002 | |
| White | REFERENCE | |
| Black or African American | 0.63 (0.45–0.85) | |
| American Indian or Alaska Native | 0.43 (0.06–3.08) | |
| Asian | 0.63 (0.09–4.53) | |
| Native Hawaiian or Pacific Islander | 0.83 (0.20–3.48) | |
| Unknown | 0.52 (0.37–0.73) | |
| Smoking history | 0.76 (0.56–1.02) | 0.07 |
| Recent hospitalization prior to Clostridium difficile diagnosis | P<0.001 | |
| None | REFERENCE | |
| Discharged within 12 weeks | 2.15 (1.22–3.77) | |
| Discharged within 4 weeks | 3.46 (2.18–5.48) | |
| Prior history of Clostridium difficile | 12.5 (9.21 −17.0) | P<0.001 |
Per 5-year increase of age
Other covariates tested but not included in the final multivariable model as they were not significant (p ≥ 0.1) were: ethnicity, poverty level of patient’s zip code of residence at time of H pylori diagnosis, treatment of H pylori, categorized by antibiotic regimen, PPI use (choice of PPI, dose, duration)
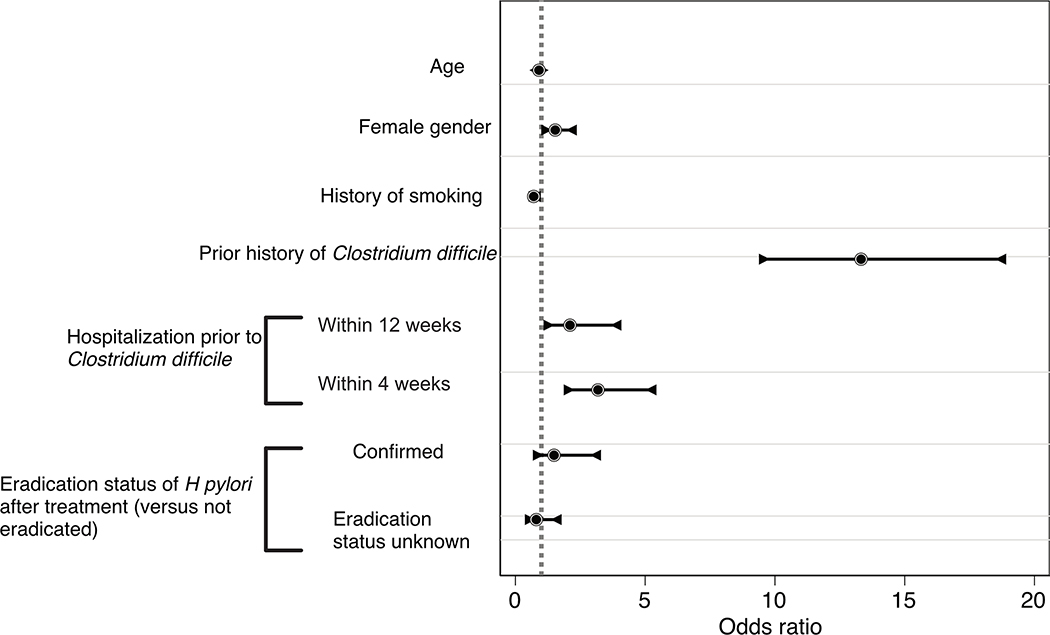
A sub-analysis of those 28,818 patients who were prescribed treatment was performed, accounting for eradication status. Of these 28,818, 933 (3.2%) had positive HP on repeat testing, while 7,541 (26.2%) were confirmed eradicated. There were 20,344 (70.6%) on whom we could not ascertain eradication status. Table 3 demonstrates the results of the multivariable analysis regarding risk factors for CDI after treatment of HP among these 28,818 patients. As compared to the larger cohort, patient demographic remained similar, and both previous CDI (OR 13.3; 95% CI: 9.38 −18.9, p<0.001) and recent hospitalization (within 12 weeks OR 2.10; 95% CI 1.08 – 4.08 and 4 weeks (OR 3.18; 95%CI: 1.86 – 5.43, p<0.001) are striking for their strength of association. Eradication status did not demonstrate that confirmation of eradication was significantly associated with future CDI (OR 1.49; 95% CI: 0.67 – 3.29). Figure 2 displays graphically the results of Table 3.
Table 3.
Risk factors for development of Clostridium difficile infection after treatment of H pylori using multivariable logistic regression model
| Odds ratio (95% CI) | P-value | |
|---|---|---|
| Age at H pylori diagnosis * | 0.91 (0.86–0.96) | P<0.001 |
| Female gender | 1.54 (1.00–2.37) | 0.05 |
| Smoking history | 0.71 (0.51–0.99) | 0.04 |
| Recent hospitalization prior to Clostridium difficile diagnosis | P<0.001 | |
| None | REFERENCE | |
| Discharged within 12 weeks | 2.10 (1.08–4.08) | |
| Discharged within 4 weeks | 3.18 (1.86–5.43) | |
| Prior history of Clostridium difficile | 13.3 (9.38 −18.9) | P<0.001 |
| Eradication confirmation of H pylori | 0.0003 | |
| Not eradicated | REFERENCE | |
| Confirmed eradicated | 1.49 (0.67–3.29) | |
| Unknown eradication status | 0.81 (0.37–1.78) |
Per 5-year increase of age
Other covariates tested but not included in the final multivariable model as they were not significant (p ≥ 0.1) were: race, ethnicity, poverty level of patient’s zip code of residence at time of H pylori diagnosis
Figure 2.
Graphical depiction of odds ratios and 95% confidence intervals based on multivariable logistic regression model evaluating risk factors for CDI after treatment of HP.
DISCUSSION
In the largest cohort of US patients with detected HP, we demonstrate that CDI after HP is rare, but not negligible at 0.74% within 3 months. Among those without recent hospitalization or prior CDI, the rate was lower, 0.37% within 3 months. The most important finding from our study was that neither treatment of HP, nor its eradication, is associated with future CDI. In multivariable analysis, we identify risk factors for CDI after HP treatment: prior history of CDI, and recent hospitalization, both of which are well-known and previously established in other studies.25–27 That younger age, White race, and female gender were associated with future CDI is interesting. Women are more likely to receive antibiotics than men, and while we controlled for HP antibiotic regimen, perhaps women have alterations in microbiota from prior (non-HP) antibiotic use, predisposing them to CDI.28 There is much recent evidence for a changing epidemiology of CDI, and these trends may be reflective of that, particularly given that older age was previously considered a strong risk factor.25 Despite quinolones being a known risk factor for CDI, we did not find that any particular antibiotic regimen was associated with future CDI.29 PPI choice, dose, and duration were not associated with CDI.
CDI after HP has been a reasonable, but theoretical, cause for concern, with previous studies limited by sample size and lack of granular data. As we note above, previous literature has generally been limited to case reports.17–19 The largest series we identified included 260 patients with ICD code diagnoses of HP with 12 subsequent cases of CDI.30 This series demonstrated that 4.6% of patients developed CDI, though two had additional antibiotic therapy prior to infection and four had hospitalization prior to infection. There was also a 3-year study period in which infection was captured, though previous literature has established that CDI is most frequent in the one to three month period after antibiotic use.31 These factors likely explain the differences between our findings.
The theoretical concern of CDI after HP could be microbiota change from the antibiotic regimen, microbiome change from HP eradication, or an otherwise un-delineated protective effect of HP against CDI. As such, we evaluated both receipt of HP treatment and subsequent eradication status, and future studies should attempt to identify the mechanism by which patients are predisposed to CDI. Because we show that previous CDI and recent hospitalization are the biggest risk factors for CDI after HP, these factors should be considered prior to prescribing therapy. For example, if a patient is hospitalized and found to have HP, in the right setting with ensured follow-up, HP eradication therapy could be delayed for a period, though future studies and guidelines should comment on the appropriateness of this strategy. It is possible that clinicians are already taking CDI into account during HP therapy. For example, we demonstrate that while patients with future CDI were more likely be prescribed an HP eradication regimen that contained metronidazole, metronidazole was not associated with future CDI. We hypothesize that this is due to clinical judgement: i.e. a clinical may elect for a metronidazole containing HP regimen due to a patient’s history of CDI or recent hospitalization.
Limitations of this study include its retrospective nature and the inherent limitations of retrospective studies in determining causality. There are measurement issues, including patients receiving care outside the VHA, and incomplete information. While the VHA is among the largest comprehensive electronic medical records in the US, it is not complete, and not linked with outside electronic medical records. As such, if a patient was diagnosed with CDI outside of the VHA, or received treatment or eradication testing outside the VHA, we would not capture this. These limitations could decrease the apparent incidence of CDI, or its association with HP treatment / eradication. When compared to those with persistent eradication, the point estimate among patients with confirmed eradication suggested a possible increased risk (OR 1.49, 95% CI: 0.67–3.29), but the confidence interval crossed 1. This may be an issue related to sample size and a limitation in power, or may simply reflect a lack of an association. However, the VHA is a longitudinal and comprehensive system, previously validated among many diseases and used widely in epidemiologic studies, and that we detect significant differences between those who develop CDI and those who do not (including reproducing previously established risk factors), lends validity to our cohort. Our success rate, while high, has been previously noted in the cohort, and is similar to published trial data on HP eradication therapy, further lending validity to our cohort.32, 33 Another limitation is that we did not have complete and long-term PPI information. While we were able to identify patients with prescription of PPI therapy outside of the HP eradication period, we were unable to analyze duration of use of long-term PPI (which is outside the scope of this manuscript) and since PPIs are a commonly available over the counter medication, we cannot reliably conclude there is no association between PPI therapy and CDI, a question that has been previously raised.34 Similarly, we do not have information on other antacids, such as Pepto-Bismol or other acid suppressing agents. As such, while we can conclude that PPI choice as part of HP therapy does not appear to be associated with CDI, our suggestion that long-term PPI is not associated with CDI should only be considered hypothesis generating.
Our strengths include the size of our cohort, which is the largest cohort of patients with detected HP in the US, and our ability to identify granular data. For example, we did not need to rely on administrative codes for CDI, but were able to confirm true positivity by evaluating testing for CDI. We were able to identify regimens of HP eradication therapy, to identify if particular regimens or antibiotics were associated with CDI. Previous literature has been limited to case reports or series. Our study, with longitudinal and granular data of high fidelity among almost 40,000 patients in the VHA provides a more complete picture of CDI after HP. It suggests that HP treatment does not increase the risk of CDI on its own, and reaffirms what is known re CDI: that prior history of CDI and recent hospitalization are the most important factors. Though this should be confirmed in prospective studies, our findings suggest that HP should be continue to be treated when detected, given that it likely modifies future gastrointestinal disease risks, including peptic ulcer disease, gastric lymphoma and adenocarcinoma.
Supplementary Material
Acknowledgments
Grant support:
Shria Kumar, MD is supported by an NIH training grant (5 T32 DK 7740-22)
Disclosures:
Shria Kumar, MD: Travel (Boston Scientific Corporation, Olympus Corporation)
David C. Metz, MBBCh: Consulting (Takeda, Lexicon, AAA. Novartis), Grant Support (Lexicon, Wren Laboratories, Ipsen, AAA)
David E. Kaplan, MD, MSc: Research grant support (Gilead, Bayer)
David S. Goldberg, MD, MSCE: Research grant support (Gilead, Merck, AbbVie, Zydus)
Abbreviations
- CDI
Clostridium difficile infection
- CDW
Corporate Data Warehouse
- HP
Helicobacter pylori
- ICD
International Classification of Diseases
- VHA
Veterans Health Administration
REFERENCES
- 1.Gerrits MM, van Vliet AH, Kuipers EJ, et al. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis 2006;6:699–709. [DOI] [PubMed] [Google Scholar]
- 2.Crowe SE. Helicobacter pylori Infection. N Engl J Med 2019;380:1158–1165. [DOI] [PubMed] [Google Scholar]
- 3.Fallone CA, Moss SF, Malfertheiner P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019;157:44–53. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol 2018;16:992–1002 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212–239. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 7.Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst 2006;98:1445–52. [DOI] [PubMed] [Google Scholar]
- 8.Nyren O, Blot WJ. Helicobacter pylori infection: mainly foe but also friend? J Natl Cancer Inst 2006;98:1432–4. [DOI] [PubMed] [Google Scholar]
- 9.Hansen S, Melby KK, Aase S, et al. Helicobacter pylori infection and risk of cardia cancer and non-cardia gastric cancer. A nested case-control study. Scand J Gastroenterol 1999;34:353–60. [DOI] [PubMed] [Google Scholar]
- 10.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila) 2008;1:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadley C The infection connection. Helicobacter pylori is more than just the cause of gastric ulcers--it offers an unprecedented opportunity to study changes in human microecology and the nature of chronic disease. EMBO Rep 2006;7:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaser MJ. Our missing microbes: Short-term antibiotic courses have long-term consequences. Cleve Clin J Med 2018;85:928–930. [DOI] [PubMed] [Google Scholar]
- 13.MJ B Missing microbes: how the overuse of antibiotics is fueling our modern plagues. New York: Henry Holt and Company, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandlund J, Estis J, Katzenbach P, et al. Increased Clinical Specificity with Ultrasensitive Detection of Clostridioides difficile Toxins: Reduction of Overdiagnosis Compared to Nucleic Acid Amplification Tests. J Clin Microbiol 2019;57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashiru-Oredope D, Sharland M, Charani E, et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart--Then Focus. J Antimicrob Chemother 2012;67 Suppl 1:i51–63. [DOI] [PubMed] [Google Scholar]
- 17.Nawaz A, Mohammed I, Ahsan K, et al. Clostridium difficile colitis associated with treatment of Helicobacter pylori infection. Am J Gastroenterol 1998;93:1175–6. [DOI] [PubMed] [Google Scholar]
- 18.Trifan A, Girleanu I, Cojocariu C, et al. Pseudomembranous colitis associated with a triple therapy for Helicobacter pylori eradication. World J Gastroenterol 2013;19:7476–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archimandritis A, Souyioultzis S, Katsorida M, et al. Clostridium difficile colitis associated with a ‘triple’ regimen, containing clarithromycin and metronidazole, to eradicate Helicobacter pylori. J Intern Med 1998;243:251–3. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Metz DC, Ellenberg S, et al. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiley LK, Shah A, Xu H, et al. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc 2013;20:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy CR, Avery TR, Dubberke ER, et al. Frequent hospital readmissions for Clostridium difficile infection and the impact on estimates of hospital-associated C. difficile burden. Infect Control Hosp Epidemiol 2012;33:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cioni G, Viale P, Frasson S, et al. Epidemiology and outcome of Clostridium difficile infections in patients hospitalized in Internal Medicine: findings from the nationwide FADOI-PRACTICE study. BMC Infect Dis 2016;16:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imase K, Takahashi M, Tanaka A, et al. Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiol Immunol 2008;52:156–61. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease C, Prevention. Surveillance for community-associated Clostridium difficile--Connecticut, 2006. MMWR Morb Mortal Wkly Rep 2008;57:340–3. [PubMed] [Google Scholar]
- 26.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis 2008;46 Suppl 1:S12–8. [DOI] [PubMed] [Google Scholar]
- 27.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 2006;12:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder W, Sommer H, Gladstone BP, et al. Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J Antimicrob Chemother 2016;71:1800–6. [DOI] [PubMed] [Google Scholar]
- 29.Yip C, Loeb M, Salama S, et al. Quinolone use as a risk factor for nosocomial Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol 2001;22:572–5. [DOI] [PubMed] [Google Scholar]
- 30.Mohammad Arsalan Siddiqui OS, Umair Iqbal, Osama Siddique, Syed-Mohammed Jafri, Mark Blumenkehl . Incidence of Clostridium Difficile in Patient Treated with Helicobacter Pylori Eradication Therapy. Gastroenterology 2017;Volume 152. [Google Scholar]
- 31.Hensgens MP, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012;67:742–8. [DOI] [PubMed] [Google Scholar]
- 32.Alhooei S, Tirgar Fakheri H, Hosseini V, et al. A Comparison between Hybrid and Concomitant Regimens for Helicobacter Pylori Eradication: A Randomized Clinical Trial. Middle East J Dig Dis 2016;8:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol 2014;5:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mezoff EA, Cohen MB. Acid suppression and the risk of Clostridium difficile infection. J Pediatr 2013;163:627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.