Fig. 1.
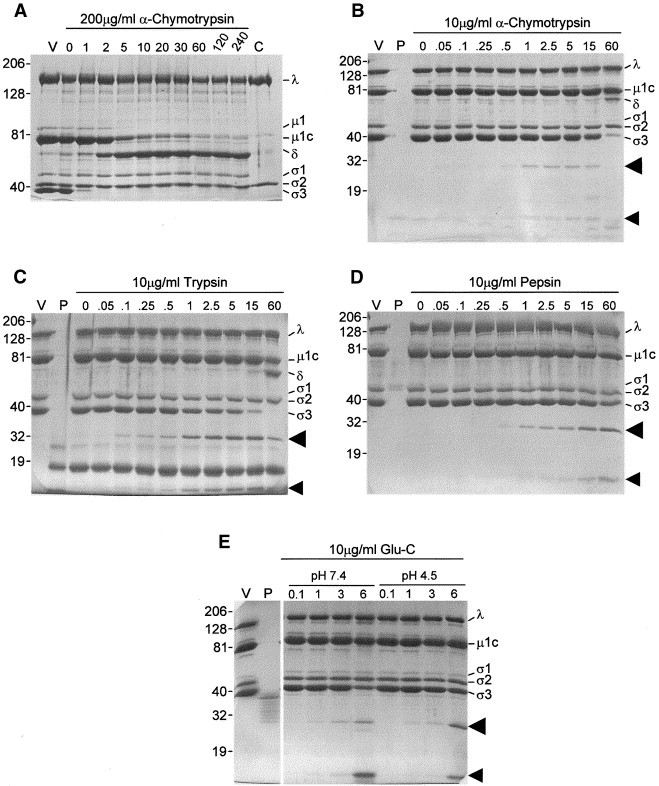
Kinetics of virus digestion. Aliquots of gradient-purified T1L virions were diluted to concentrations of 7.3–8.6 × 1011 particles/μl in appropriate buffers (pH 7.4 for α-chymotrypsin, trypsin, and S. aureus V8 (Glu-C); or pH 4.5 for Glu-C; or pH 3.0 for pepsin), digested with either (A) 200 μg/ml α-chymotrypsin, or (B) 10 μg/ml α-chymotrypsin, (C) 10 μg/ml trypsin, (D) 10 μg/ml pepsin, or (E) 10 μg/ml Glu-C for the indicated periods of time (minutes for (A–D); hours for (E)) at 37°C. Reactions were stopped by chilling and addition of phenylmethylsulfonyl fluoride to a final concentration of 5 mM to α-chymotrypsin reactions, by addition of soybean trypsin inhibitor to a final concentration of 125 μg/ml to trypsin reactions, by adjustment of pepsin reactions to a pH of 7.5, or by chilling Glu-C reactions. One-quarter volume of 5× electrophoresis sample buffer was added and peptides from about 1 × 1011 particles were resolved in either a 4–16% exponential gradient (A) or a 6–16% linear gradient (B–E) mini-SDS–PAGE (8 × 10 × 0.04 cm) at 150–180 V for 60 min. Gels were fixed and stained with Coomassie brilliant blue R-250. V: gradient-purified virions; P: 150 ng of indicated protease; C: gradient-purified core particles, separately prepared. Molecular weights, determined from coelectrophoresis of Kaleideoscope Rainbow Marker, are indicated to left of each gel. Virion proteins (λ, μ, and σ1–σ3), μ1C digestion product δ, and potential σ3 digestion products (indicated by arrowheads) are indicated to the right of each gel.
