Fig.5. Truncation of the cytoplasmatic tail results in increased surface expression of iRhom2.
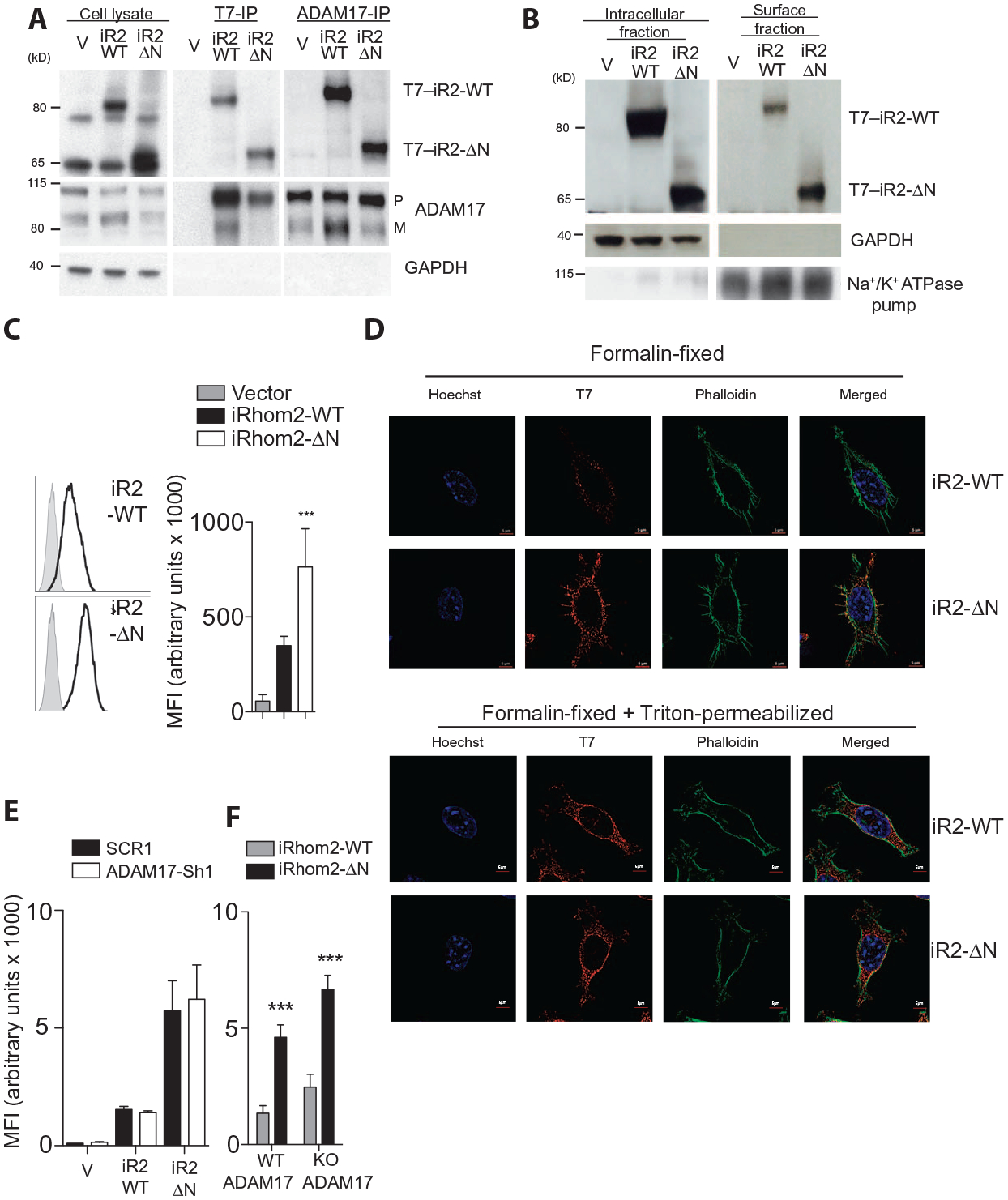
(A) Immunoprecipitation (IP) for T7 or ADAM17 followed by immunoblotting for the same in lysates from L-929 cells stably expressing T7-tagged WT or truncated iRhom2. Blot is representative of three experiments. (B) Immunoblotting for iRhom2 using a T7 antibody in intracellular and cell surface fractions from L-929 cells stably expressing WT or truncated iRhom2. Blot is representative of three experiments. (C) Surface abundance of iRhom2, determined using an antibody against T7, on stably transfected L-929 cells (n = 8). (D) Immunocytochemistry for iRhom2 using T7 antibodies (Cy3), phalloidin–fluorescein isothiocyanate (FITC), and Hoechst staining in stably transfected L-929 cells, fixed, and/or permeabilized as indicated (n ≥ 3 experiments). (E and F) Flow cytometry analysis of MFI of the surface abundance of iRhom2 on unpermeabilized stably transfected L-929 cells expressing either scrambled or ADAM17 shRNA (n ≥ 4 experiments) (E) or immortalized WT or Adam17 knockout (KO) mouse embryonic fibroblasts (MEFs) (n = 12 experiments) (F). Data are means ± SEM from the number of experiments (n) indicated; ***P < 0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Na+/K+ ATPase, Na+- and K+-dependent adenosine triphosphatase.
