Figure 6.
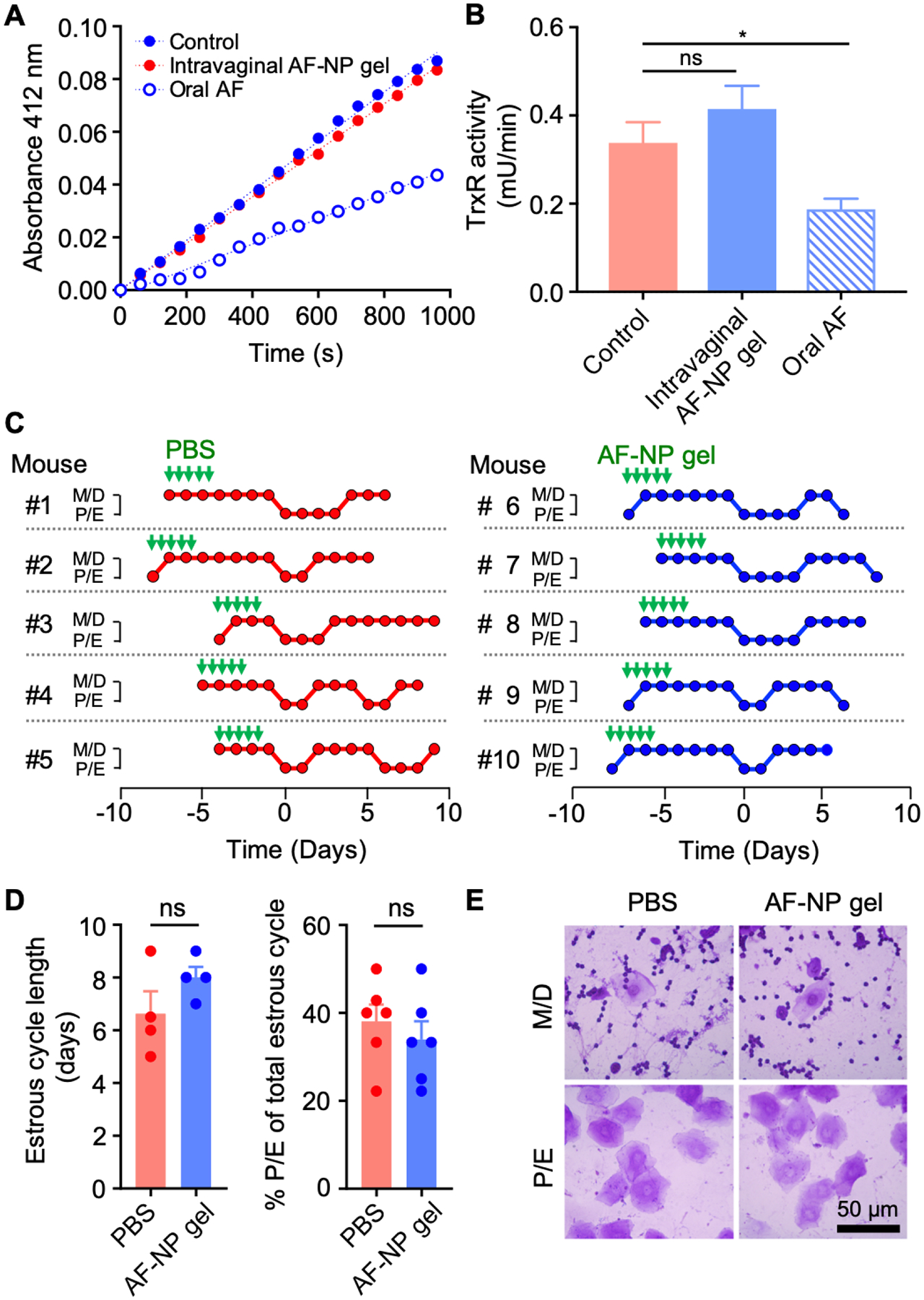
Toxicity evaluation of AF-NP gel. (A, B) Adult female BALB/c mice were given fives doses over three days of either intravaginal AF-NP gel (10 μg AF/dose) or oral unformulated AF (30 mg/kg per dose), with untreated mice as controls. Liver extracts were prepared and assayed for TrxR activity by DTNB reduction. Representative reaction kinetics are shown in panel A, and enzyme activities are shown in panel B (mean ± SEM, n=18; *p<0.05 by Kruskal-Wallis test with Dunn’s post-hoc test; ns, not significant). (C-E) Estrus cycle analysis. Adult female mice were given intravaginal AF-NP gel (10 μg/dose) for five doses over three days, or PBS as controls, as indicated by the green arrows. Estrus cycle phases were determined by daily cytological analysis of crystal violet-stained vaginal washes in individual mice (C). Metestrus and diestrus (M/D) are cytologically characterized by predominant leukocytes mixed with occasional epithelial cells, while proestrus and estrus (P/E) are characterized by >90% nucleated and/or cornified epithelial cells. Phases are lined up in the graphs for the first detected M/D to P/E transition (day 0). Cycle length was determined by the time between M/D to P/E transitions or P/E to M/D transitions, or the average of both in any given mouse. The percentage of the P/E phase within the total cycle was calculated by days in the P/E phase divided by the total cycle length (D; mean ± SEM, n=5/group; ns, not significant by Mann-Whitney test). (E) Representative photographs of stained vaginal smears for mice treated with 5 x doses of AF-NP gel or PBS as a control. No morphological abnormalities were observed after AP-NP gel administration.
