Figure 1: Patient demographics. Clinical trial conduct with regard to donors, donor bone marrow infusion, and maintenance immunosuppressive regimen.
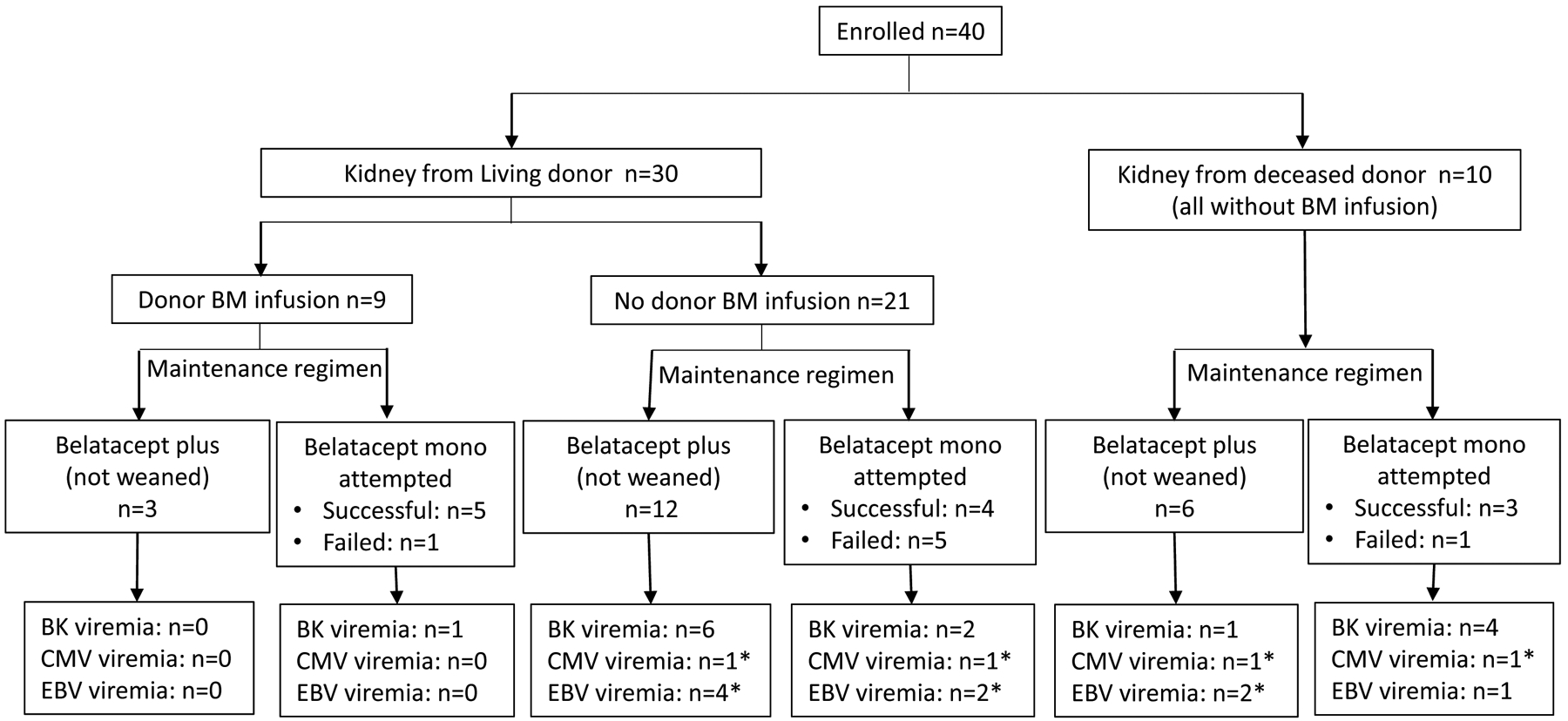
All patients received alemtuzumab induction followed by belatacept maintenance therapy, plus sirolimus (or MMF in patients who were intolerant to sirolimus). The opportunity to wean off sirolimus (or MMF) followed by belatacept monotherapy was offered to patients who met prespecified criteria at 12 months posttransplantation. Unsuccessfully weaning was determined by evidence of rejection (clinical or subclinical) on biopsy. No patient had clinical rejection in the first year. * All episodes of CMV or EBV were transient low positive defined as values < 300 copies/mL.
