Fig. 1. Characterization of inactivated CVB1–6 viruses, experimental setup, and vaccine safety and immunogenicity in C57BL/6J mice.
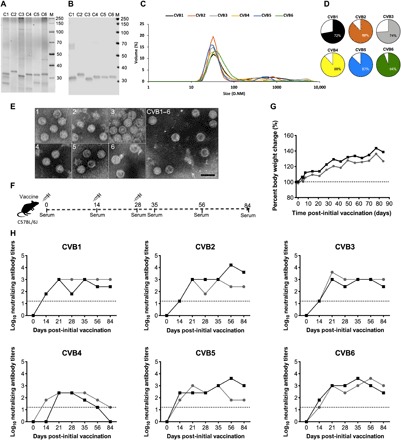
CVB1–6 viruses were propagated in Vero cells, purified, inactivated with formalin, and then characterized as described in Materials and Methods. (A) Analysis of CVB1–6 total protein and virus protein content (2 μg of virus per well) by SDS-PAGE followed by (B) Western blot analysis using the in-house produced rat monoclonal antibody 3A6, which binds to the CVB1–6 viral capsid protein VP1. (C) DLS analysis of the inactivated CVB1–6 serotypes. D.NM is the diameter in nm of the particles. (D) Volume distribution (percentages) of the most prominent particle populations in the vaccine preparations as measured by DLS analysis. (E) TEM analysis of individual inactivated CVB1–6 vaccine components (serotypes are indicated by the number in the top left-hand corner of each image) and the vaccine mix (CVB1–6). Scale bar, 50 nm. (F) Experimental setup for the C57BL/6J CVB1–6 vaccine studies. (G and H) Two female C57BL/6J mice (age 7.8 weeks), represented by individual lines, were vaccinated on days 0, 14, and 28 with CVB1–6 vaccine (1-μg dose of each serotype, 150 μl, interscapularly). (G) Percentage body weight change from the first vaccination (day 0); the dotted line indicates the weight on day 0. (H) CVB nAB titers in the serum of mice after vaccination. The dotted lines show the positivity cutoff for the method. C1, CVB1; C2, CVB2; C3, CVB3; C4, CVB4; C5, CVB5; C6, CVB6.
