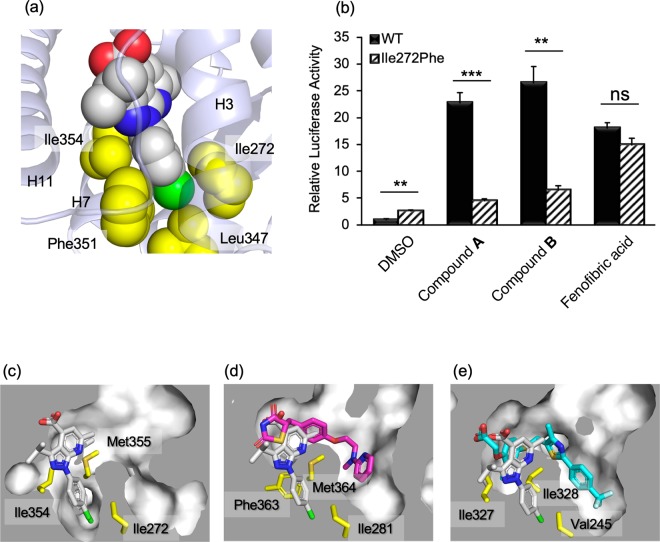
Figure 5.
Interactions of the p-halophenyl substituent of the 1H-pyrazolo-[3,4-b]pyridine derivative to the hydrophobic cleft between helices 3 and 7. (a) Compound A is represented by a space-filling model (C: white, N: blue, O: red, and Cl: green). The sidechains of interacting amino acid residues are depicted as yellow space-filling models. (b) Transactivation of WT and Ile272Phe mutant of hPPARα LBD by compounds. Cells transfected with the reporter plasmid were treated with 1 μM of compounds A, B, 10 μM of fenofibric acid, or 0.1% dimethyl sulfoxide (vehicle). The free acid form of compound A was used in this assay. Values are expressed as the fold-induction compared to the vehicle, which was set to 1. For all graphs, error bars indicate the mean ± SE of three independent measurements. Significant differences between the values of WT and Ile272Phe were determined using Student’s t-test (*P < 0.05, **P < 0.01, and ***P < 0.001). (c) Close-up views of interactions between PPARα LBD and compound A (white), (d) PPARγ LBD and rosiglitazone (magenta), and (e) PPARβ/δ LBD and GW501516 (cyan). Cross-sections of the binding pockets are depicted with a gray surface. The binding position of compound A is shown in (d) and (e). These figures have been created with PyMol 2.3 (Schrödinger LLC, https://pymol.org).