Figure 3.
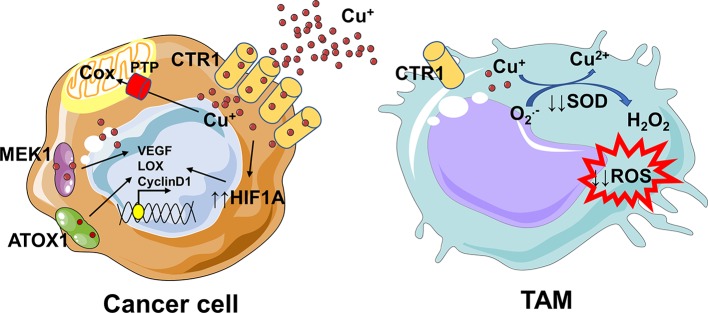
Copper addiction of cancer cells could prevent the pro-inflammatory phenotype of macrophages. The increased copper flow via CTR1 is followed by loading onto the copper chaperone ATOX1, which acts as a copper-dependent transcription factor promoting cyclin D1 expression and cell replication. Since copper is essential for the activity of proteins, like cytochrome c oxidase (Cox), involved in the mitochondrial electron transport chain, mitochondria rely on the phosphate carrier SLC25A3 (PTP) for its uptake. Copper not only binds to proteins directly involved in cancer progression, such as MEK1, but also indirectly modulates their expression or activation. Copper inhibits prolyl hydroxylase thus stabilizing HIF-1α and increasing the transcription of several angiogenic genes (e.g., ceruloplasmin and VEGF) and genes involved in the epithelial to mesenchymal transition (e.g., LOX). Copper is essential for sustaining the pro-inflammatory phenotype of macrophages; indeed, as a component of the SOD enzyme which catalyzes the production of H2O2 from superoxide, it contributes to the ROS-dependent killing capacity of macrophages. The removal of copper from microenvironment by cancer cells might drive the polarization of TAMs toward a pro-tumoral M2-like phenotype. In parts the figures are based on speculations and have been prepared by assembling in-house built cellular metabolic pathway outlines with a modified and adapted version of BioRender images.