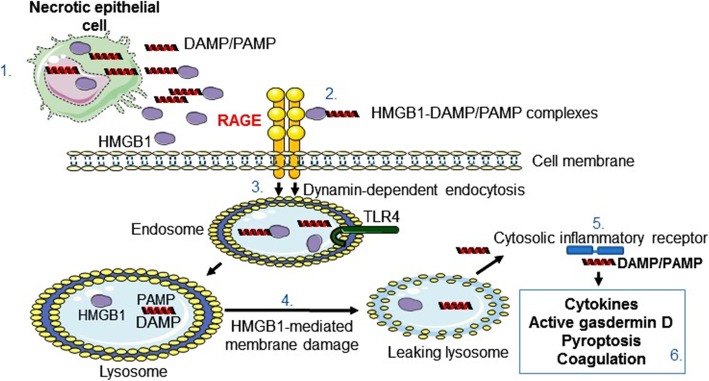
Fig. 1.
Inflammation induced by HMGB1-partner molecule complexes. Necrotic cells release DAMP and PAMP molecules extracellularly where they form complexes with HMGB1 released from dying or activated cells (1); these complexes bind to RAGE abundantly expressed in the lungs (2); and get endocytosed to endosomes having TLR receptors including TLR4 which may be activated by HMGB1 (3); HMGB1 and partner molecules translocate to lysosomes, where HMGB1 acts like a detergent under the acidic conditions and disrupts the lysosomal membrane enabling HMGB1-partner molecules access to the cytosol (4); the translocated molecules bind and activate reciprocal cytoplasmic receptors generating inflammasome activation and additional proinflammatory events (5); the subsequent outcome production and extracellular release of cytokines via pore formation in the cell surface membrane accomplished by oligomerized gasdermin D. The final outcome is pyroptosis. Active gasdermin D also rotates and translocates phosphatidylserine molecules to the outside of the cell surface membrane and induces tissue factor on endothelial cells. This biology initiates coagulation (6)