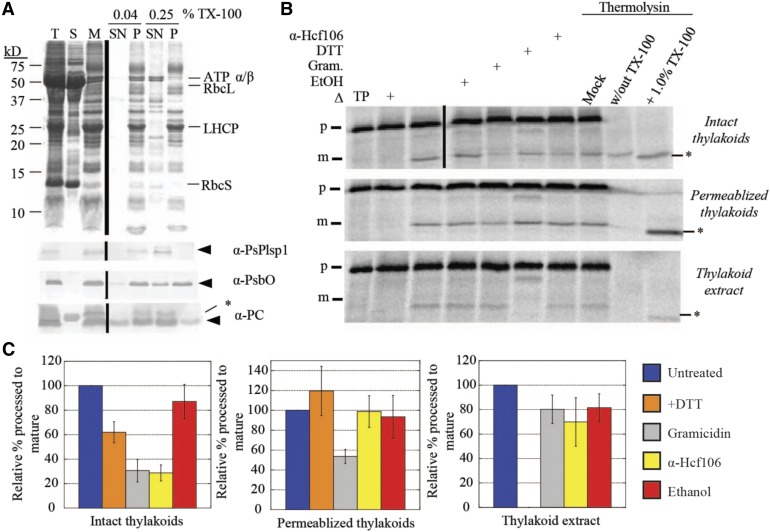
Figure 5.
Thylakoid Membrane-Bound Processing Activity Is Insensitive to DTT.
(A) SDS-PAGE analysis of Pea chloroplast fractions. Proteins were detected by Coomassie Brilliant Blue staining (top panel) or immunoblotting (bottom three panels) with the antibodies indicated at the right of each panel. Arrowheads indicate proteins of interest, and asterisks indicate nonspecific immunoreactive bands. A volume equivalent to 4 μg chlorophyll was loaded in each lane. T = total chloroplasts; S = soluble fraction; M = membranes; SN = supernatant; P = pellet.
(B) Processing activity in Pea chloroplast membrane fractions depicted in (A). The 4 μg chlorophyll equivalents of each of the three fractions were pretreated on ice for 30 min with reaction buffer (control), 1% ethanol (EtOH), 30 μM gramicidin (Gram) in 1% ethanol, 50 mM DTT, or the α-Hcf106 antibody. As a control, one sample of each chloroplast fraction was heat-inactivated (Δ) at 82°C for 10 min before adding the substrate. After 35S-Met-prPsbP was added, each reaction mixture was incubated in the dark at 28°C for 30 min. After the incubation at 28°C, three additional control reactions were treated with 0.5 mM CaCl2, thermolysin (0.1 mg/mL with 0.5 mM CaCl2), or thermolysin (0.1 mg/mL, with CaCl2) in the presence of Triton X-100 (1% v/v) for 40 min on ice. Reaction mixtures were quenched by adding an equal volume of 2× sample buffer containing 30 mM EDTA and boiling for 5 min. Samples were analyzed by SDS-PAGE and autoradiography. Asterisks indicate a degradation product that was observed in some experiments.
(C) ImageJ quantification of mature PsbP bands shown in (B). Values were normalized to the band intensity in the untreated sample and are expressed as the mean ± sd of at least three independent experiments.