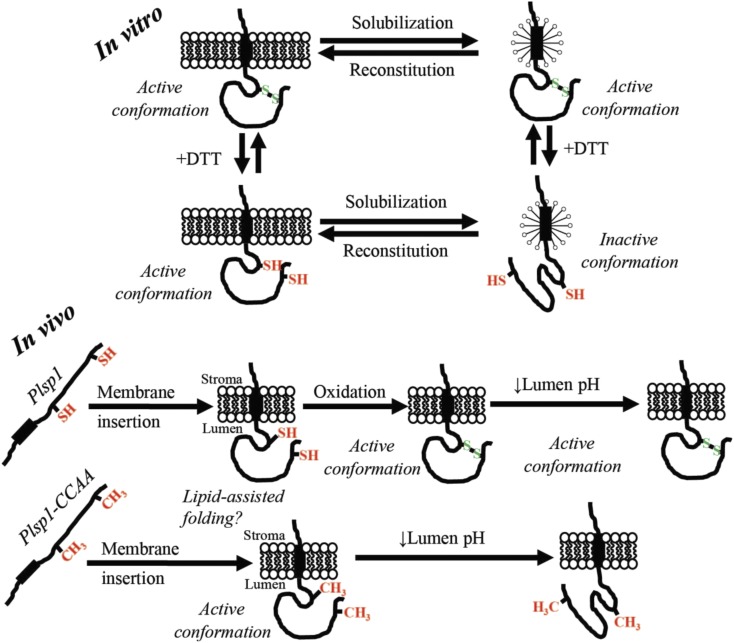
Figure 9.
In Vitro and In Vivo Models.
For in vitro, Plsp1 is solubilized in from membranes in an active form due to an intramolecular disulfide bond. Reduction of this disulfide bond causes an inactivating conformational change in detergent micelles that is prevented by association with a lipid bilayer. Reintegration of Plsp1 into a bilayer causes reconstitutive folding back into the active state. For in vivo, Plsp1 is targeted to thylakoids in a reduced form. Once the catalytic domain traverses the membrane, reconstitutive folding into the catalytically active form is assisted by bilayer lipids. In the case of the wild-type protein, oxidation to form the disulfide bond then takes place and stabilizes the structure. Structural stabilization by the disulfide bond allows Plsp1 to remain optimally active amid fluctuations in the lumen pH.