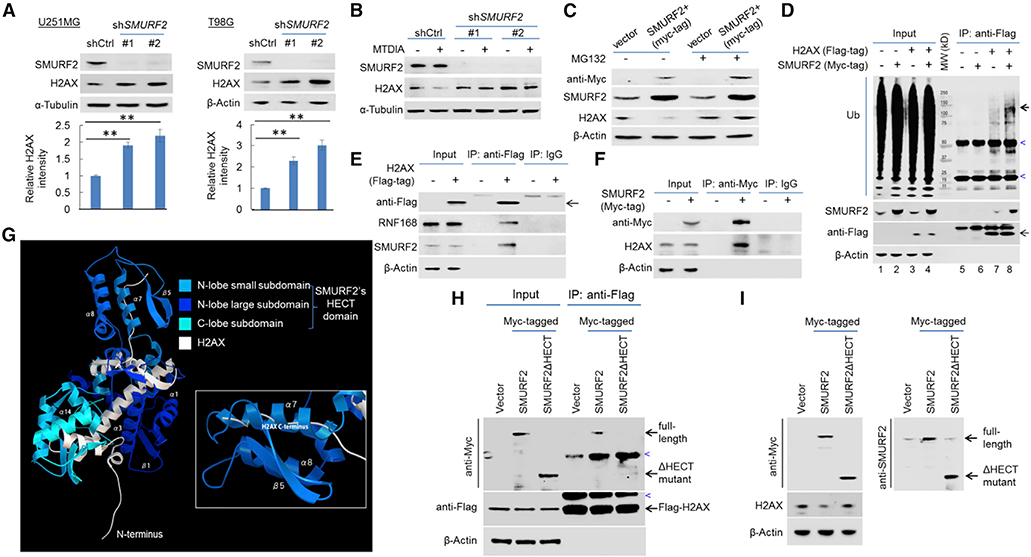
Figure 6. SMURF2 Negatively Regulates H2AX Proteostasis.
(A) Immunoblot detection of H2AX in U251MG and T98G cell lines without or with SMURF2 knockdown (SMURF2 knockdown was confirmed by an anti-SMURF2 immunoblot). The quantification of the relative abundance of H2AX in each lane is shown in the bottom panels.
(B) U251MG cells, without or with SMURF2 knockdown, were treated with vehicle control or with MTDIA (3 μM, 24 h), and immunoblotting was performed to determine the abundance of H2AX.
(C) Immunoblot detection of H2AX in U251MG cells without (vector) or with exogenous Myc-tagged SMURF2 overexpression (SMURF2+), under the treatment of DMSO or MG132 (10 μM, 24 h).
(D) Control U251MG cell line and its derivative lines expressing exogenous FLAG-tagged H2AX, Myc-tagged SMURF2, or both were used for anti-FLAG co-immunoprecipitation and immunoblot analysis to determine the poly-ubiquit ination of H2AX following overexpression of SMURF2. Note the stronger ubiquitination signal (bold arrow) in lane 8 compared to lane 7. The heavy bands (marked by blue arrowhead) were likely due to the antibody used for IP or non-specific immune blot signaling.
(E) U251MG cells expressing exogenous FLAG-tagged H2AX were used for co-immunoprecipitation with control IgG or with an anti-FLAG antibody, and immunoblotting was performed.
(F) U251MG cells expressing exogenous Myc-tagged SMURF2 were used for co-immunoprecipitation with control IgG or with an anti-Myc antibody, and immunoblotting was performed.
(G) A simulation-based model depicts the interaction of SMURF2’s HECT domain with H2AX.
(H) FLAG-tagged H2AX expressing U251MG cell lines, expressing exogenous Myc-tagged SMURF2, or SMURF2ΔHECT mutant were used for anti-FLAG co-immunoprecipitation (co-IP) and immunoblots with indicated antibodies.
(I) The same set of cell lines were used for immunoblots with indicated antibodies. Blue arrowheads denote non-specific bands (likely due to antibodies used for IPs).
Note that for co-immunoprecipitations in (D)–(F) and (H), cells were treated with MG132 (10 μM, to ensure equal H2AX abundance) 1 day before the co-immunoprecipitation experiments. Error bars represent mean ± SD.