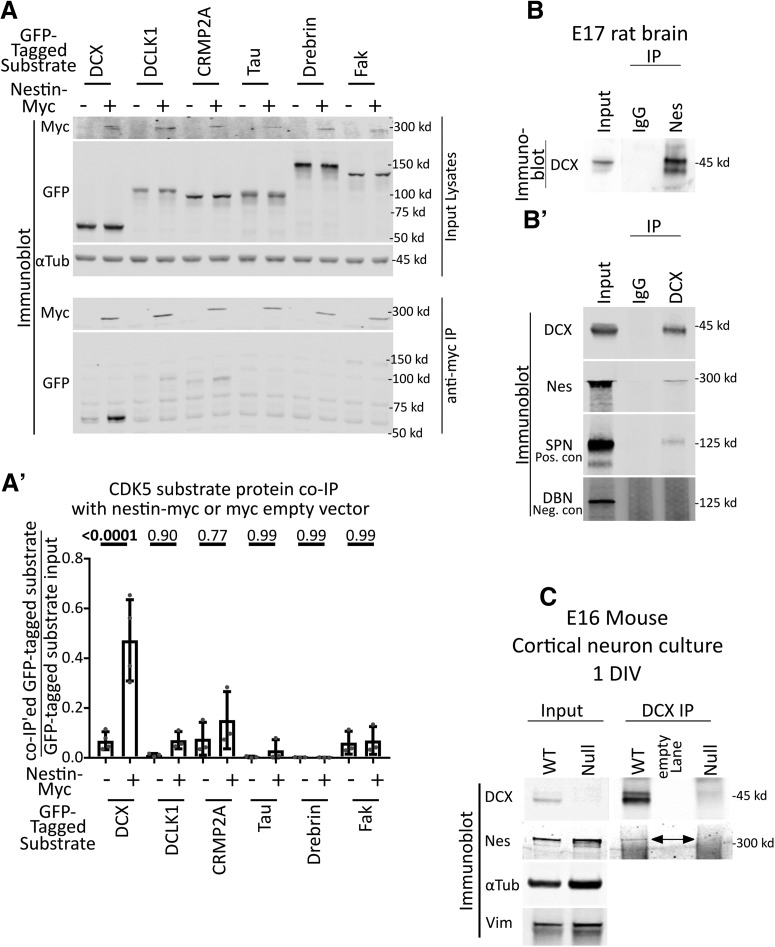
Figure 3.
Nestin forms a complex specifically with DCX. A, Candidate screen for cdk5 substrates that interact with nestin by coimmunoprecipitation. Six different GFP-tagged cdk5 substrates were expressed with or without nestin-myc in HEK293 cells and subjected to immunoprecipitation with anti-myc antibody. An empty vector (EV) was used as a negative control. Top, Input lysates are shown. α-Tubulin is used as a loading control. Bottom, Anti-myc IPs are probed against myc or GFP. Only GFP-DCX coimmunoprecipitates with nestin-myc. A′, Densitometry analysis of each coimmunoprecipitated substrate relative to its input. Statistical comparisons (ordinary one-way ANOVA with Sidak's correction) were performed between the EV myc IP compared with nestin-myc IP. Errors bars indicate SD. n = at least 3 for each condition. p values are indicated above the graph. p values of <0.05 are indicated in bold. B, B′, IP of endogenous nestin and DCX complexes from brain. B, DCX coimmunoprecipitation with anti-nestin antibody from E17 rat brain compared with a control IgG. B′, Nestin coimmunoprecipitation with anti-DCX antibody from E17 rat brain compared with a control IgG. As a positive control, DCX IPs were probed against SPN. Drebrin serves as a negative control. C, Anti-DCX immunoprecipitation from 1 DIV cortical EXVI mouse neurons results in coimmunoprecipitation of nestin from WT neurons, but not from Dcx KO (null) neurons, demonstrating specificity of the IP. The double-headed arrow points to the band corresponding to nestin. No anti-DCX immunoreactivity is seen in the Western blots of DCX-null neurons compared with WT, also demonstrating specificity of the antibody. α-Tubulin and vimentin immunoblots are shown as loading controls.