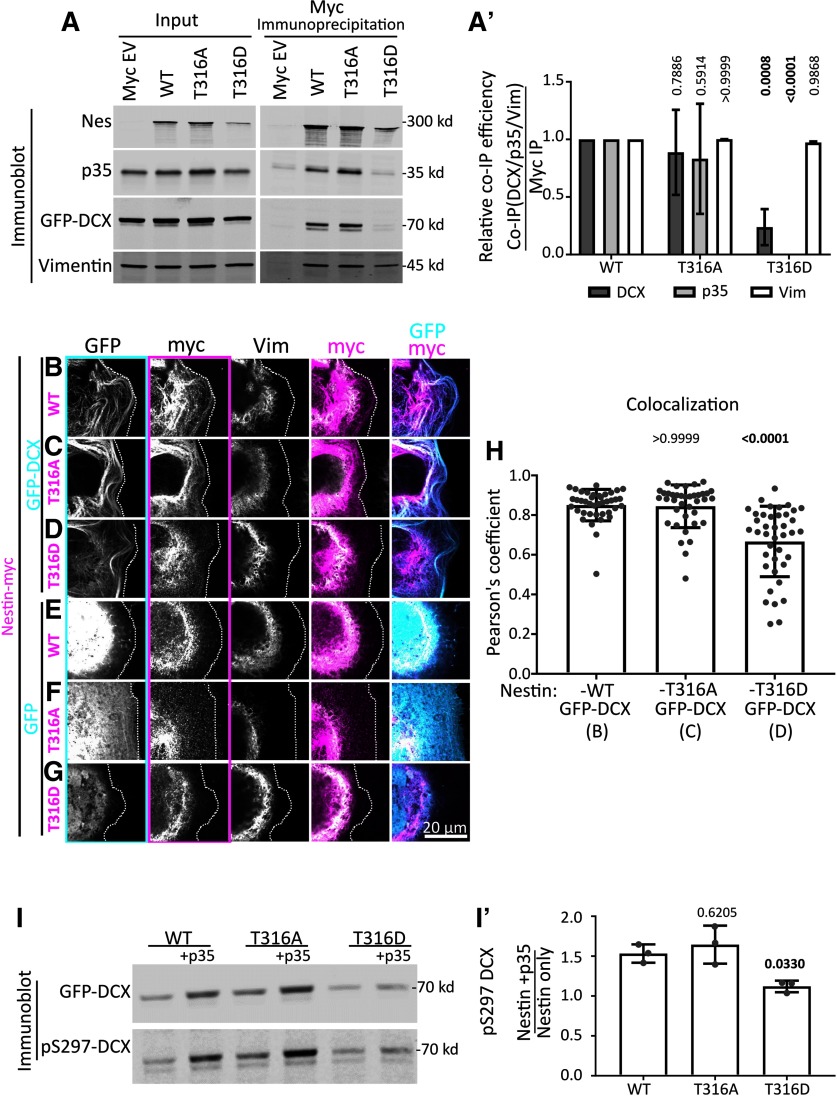
Figure 7.
The T316D mutation in nestin affects p35/DCX binding and DCX phosphorylation, but not incorporation into vimentin filaments. A, Point mutants in nestin (T316A, phospho-dead; or T316D, putative phospho-mimetic) were tested for binding to p35, DCX, and vimentin by coimmunoprecipitation from HEK293 cells. Inputs (left) and corresponding coimmunoprecipitation of vimentin, GFP-DCX, and p35 after anti-myc IP (right). The p35 and DCX/vimentin panels are combined from the same experiment, conducted from two different plates of cells. The blots are qualitatively similar for both sets, and were combined into a single figure for simpler viewing. A′, Densitometry quantification of coimmunoprecipitation efficiency (coimmunoprecipitation/IP) from the blots in A. T316D-nestin pulls down greatly reduced levels of p35 and DCX compared with WT nestin. Coimmunoprecipitation of p35 and DCX by T316A-nestin is not significantly different compared with WT nestin. Each mutant coimmunoprecipitates vimentin similarly to WT. Means of n = 3 experiments for each IP condition. Error bars indicate SD. Data were analyzed with parametric two-way ANOVA test with Tukey's correction. p values shown above each condition compared with the corresponding WT-Nestin coimmunoprecipitation. p values <0.05 are in bold and are considered significant. B–G, Confocal images of Cos7 cells transfected with GFP-DCX (C-E) or GFP (F–H) cotransfected with WT nes-myc (C,F) or mutant nestin (T316A: D,G; T316D: E,H), as indicated. The transfected cell is outlined with a dotted line. Individual black and white channels are shown, as well as merges with GFP/myc and myc/vimentin channels. Outlines of black and white images indicate the color used in the composite images. WT nestin and the 316A-nestin mutant colocalize with DCX MT bundles. However, nestin is associated with vimentin when expressed without DCX or when the DCX-binding-deficient T316D-nestin is expressed, even in the presence of DCX. H, Pearson's coefficient of conditions represented in B–D (as indicated below the x-axis labels). N = 38-40 cells from three independent experiments are shown with the mean. Error bars indicate SD. The WT nestin with DCX condition has the same values from Figure 4F. Data were analyzed with nonparametric Kruskal-Wallis one-way ANOVA test with Dunn's correction, with each p value shown above each condition compared with GFP-DCX with WT Nes-myc (first lane). p values <0.05 and are in bold and are considered significant. The Pearson coefficient mean for Nes-T316A with DCX is not significantly different that Nes-WT with DCX. However, Nes-T316D with DCX has a significantly (p = 0.0001) lower Pearson coefficient mean than Nes-WT with DCX. I, Nestin-dependent cdk5 phosphorylation of DCX in HEK293 cells. I′, Densitometry quantification of the blot in B showing the relative levels of pS297-DCX phosphorylation with expression of each mutant. Each value represents the relative phosphorylation of DCX when expressed with nestin and p35, compared with just nestin expression. T316D-nestin does not augment p35/CDK5-mediated DCX phosphorylation. Error bars indicate SD. Each condition was compared with WT nestin condition (one-way ANOVA with Dunnett's correction). N = 3 independent experiments. Bold p values are <0.05 and are considered significant.