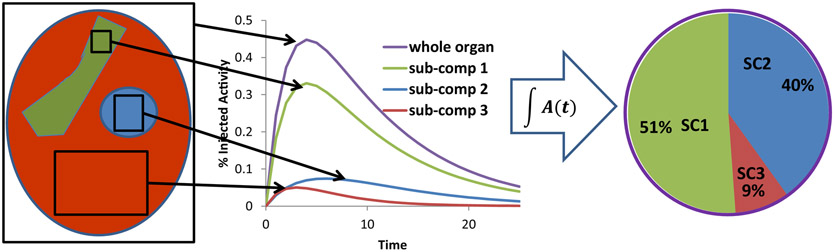
Figure 7.
Illustration of the macro to micro methodology. The methodology is founded on the ability to collect pharmacoknetics for an entire organ as well as relevant critical subcompartments of the organ in a preclinical model. This information may be used to establish apportionment factors for whole-organ measurements made in humans by quantitative imaging. The approach may be validated by assessing the variability of the apportionment factors for different pre-clinical models and also by showing within any particular model that the approach can be used to predict toxicity induced by sub-compartmental localization of the αRPT.