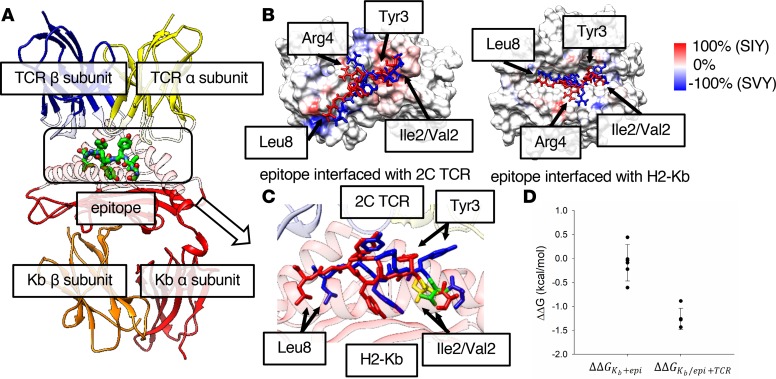
Figure 2. Modeling highlights differences in SIY and SVY binding to MHC and TCR.
(A) Structure of the H2-Kb/epitope/2C TCR complex. The α and β chains of 2C TCR and H2-Kb are represented by ribbon models in yellow, blue, red, and orange, respectively, and are transparent at the epitope interface. The epitope in the box at the center with a dotted line is represented by a stick model. (B) The difference in contact frequency between the SIY and the SVY epitopes on the 2C TCR-epitope (left) and the Kb-epitope (right) interfaces. The SIY and the SVY epitopes are colored in red and blue, respectively. The surfaces of TCR and Kb proteins are colored from blue to red, as the difference of the contact frequency is changed from –100% (SVY) to 100% (SIY). The red color indicates that protein atoms contact the SIY epitope more often than the SVY epitope, while the blue color indicates the opposite. (C) A zoomed-in image of the binding poses of the SIY (red) and the SVY (blue) epitopes, which are interfaced with H2-Kb and 2C TCR on the top and the bottom sides of the figure. The structure of each epitope is taken from the frame at the center of the most populated cluster in the MD trajectory. Ile2 of the SIY epitope and Val2 of the SVY epitope are colored in yellow and green, respectively. The α and β chains of 2C TCR and the α chain of H2-Kb are represented by ribbon models in yellow, blue, and red, respectively. (D) The relative free energies for the binding of the epitope to Kb (ΔΔGKb+epi), and the binding of TCR to the Kb-epitope complex (ΔΔGKb/epi+TCR), between the SIY and the SVY epitopes. Each free energy value was calculated by the FEP method.