Abstract
Sudden death characterizes the mode of demise in 30–50% of patients with chronic heart failure and a reduced ejection fraction. Occasionally, these events have an identifiable pathophysiological trigger, e.g. myocardial infarction, catecholamine surges, or electrolyte imbalances, but in most circumstances, there is no acute precipitating mechanism. Instead, adverse left ventricular remodelling and fibrosis creates an exceptionally fragile and highly vulnerable substrate, which can be characterized using the model developed in theoretical physics of ‘self-organizing criticality’. This framework has been applied to describe the genesis of avalanches, nodes of traffic congestion unrelated to an accident, the abrupt system-wide failure of electrical grids, and the initiation of cancer and neurodegenerative diseases. Self-organizing criticality within the ventricular myocardium relies on complex adaptations to progressive stress and stretch, which evolve inevitably to an abrupt end (termed ‘cascading failure’), even though the rate of deterioration of the underlying disease process has not changed. The result is acute circulatory collapse (i.e. sudden death) in the absence of an identifiable triggering event. Cascading failure in a severely remodelled or fibrotic heart can become manifest electrically as a first-time ventricular tachyarrhythmia that is responsive to the shock delivered by an implantable cardioverter-defibrillator (ICD). Alternatively, it may present as an acute mechanical failure, which is manifest as (i) asystole, bradyarrhythmia, or electromechanical dissociation; or (ii) incessant ventricular fibrillation that persists despite repetitive ICD discharges; in both instances, the sudden deaths cannot be prevented by an ICD. This conceptual framework explains why anti-remodelling and antifibrotic interventions (i.e. neurohormonal antagonists and cardiac resynchronization) reduce the risk of sudden death in patients with heart failure in the absence of an ICD and provide incremental benefits in those with an ICD. The adoption of anti-remodelling and antifibrotic treatments may explain why the incidence of sudden death in clinical trials of heart failure has declined dramatically over the past 10–15 years, independent of the use of ICDs.
Keywords: Heart failure, Sudden death, Implantable cardioverter-defibrillator, Neurohormonal antagonists
Introduction
Cardiac arrest is the mode of demise in 30–50% of patients with heart failure and a reduced ejection fraction (HFrEF), and conversely, systolic dysfunction is a major risk factor for sudden cardiac death in the community.1 Despite their clinical importance, the mechanisms that lead to abrupt circulatory collapse have long been misunderstood. This article presents a novel framework for understanding the pathogenesis of this event.
What is sudden cardiac death in patients with heart failure?
The original definition of sudden cardiac death—i.e. demise within 1 h of the onset of new cardiac symptoms—was developed to identify the event in the general population with no known heart disease. However, this approach could not be applied to patients with HFrEF, who had ongoing symptoms and an established cardiovascular disorder. Initially, patients with HFrEF were deemed to have died suddenly if their symptoms had not recently worsened, and if another cause for cardiac arrest could not be identified.1 Yet, this approach was difficult to apply in practice. Technically, all deaths were sudden, since the patient was alive at one moment and dead at the next observation point. Therefore, the feature that distinguished instantaneous demise was not its suddenness, but its unexpectedness. If an event had not been foreseen, physicians believed that a new mechanism had emerged and had triggered the abrupt circulatory collapse.
However, if unexpectedness were the determining factor in the identification of sudden deaths, could physicians reliably gauge the degree of unexpectedness? What if a patient had end-stage HFrEF but had stabilized with intensive therapy and then died abruptly? Was the inevitability of death sufficient to negate the diagnosis of an unexpected death? Was the period of clinical stability immediately prior to the demise a clue of the emergence a new trigger or was it an irrelevant lull in an ongoing storm that had raged for months or years? Did the designation of sudden death imply the development of a new pathophysiologic mechanism? Or was sudden death an illusion that was related to an imperfect ability to adequately discern subtle changes in the evolution of HFrEF?
Despite these uncertainties, physicians began to rely on prediction models to identify patients who died suddenly. Adjudication committees in large-scale trials decided that sudden death would not be applied to a patient who had an extremely limited life expectancy due to HFrEF.2 Sudden death was identified only if patients were anticipated to survive for many months or years. If death was expected, then the demise could be ascribed to the mechanisms that were already in play. In contrast, if the death were unexpected, it was assumed that a new mechanism had emerged, even though it was rarely clinically apparent.
Do coronary ischaemic events cause sudden death?
Three decades ago, sudden deaths were typically ascribed to an acute coronary occlusion. Autopsies of patients with HFrEF who had died suddenly indicated that an acute thrombotic occlusion could be a terminal trigger.3 However, if coronary thrombosis were a common cause of sudden death, physicians might expect these events to be prevented if the inciting plaque rupture or thrombotic occlusion were averted. Yet, the use of statins to cause plaque stabilization or the use of aspirin or oral anticoagulants to prevent coronary thrombosis did not reduce the risk of sudden death in clinical trials of HFrEF (Table 1).4–7 Coronary revascularization decreased the risk of new myocardial infarctions, but the benefit on sudden death was so modest that it took years to become apparent,8 even though the utilization of implantable cardioverter-defibrillators (ICDs) was very low (only 2%). Therefore, the role of coronary thrombosis in the genesis of sudden death in HFrEF was small.
Table 1.
Effect of drug, device and surgical interventions on the risk of sudden death in patients with left ventricular systolic dysfunction
| Patient population | Background therapy | Reduction in risk of sudden death | |
|---|---|---|---|
| Drugs or surgical procedures that prevent myocardial infarction | |||
| Statins4,5 | HFrEF | Consistent use of neurohormonal antagonists, but minimal CRT and ICDs | No benefit |
| Antiplatelet and anticoagulants6,7 | HFrEF | Robust use of neurohormonal antagonists, CRT and ICDs in rivaroxaban trial | No benefit |
| Coronary artery bypass graft surgery8 | HFrEF and coronary artery disease | Consistent use of neurohormonal antagonists, but minimal CRT and ICDs | ≈25% decreased risk evident during long-term follow-up |
| Drugs or devices that favourably affect adverse left ventricular remodelling | |||
| ACEI29,30 | HFrEF and post-infarction LVD | Minimal use of neurohormonal antagonists, CRT and ICDs | No benefit in HFrEF; 20% decreased risk in post-infarction LVD |
| Beta-adrenergic receptor blockers31–33 | HFrEF and post-infarction LVD | Use of ACEI, but not other neurohormonal antagonists, CRT and ICDs | ≈25% decreased risk in post-infarction LVD; 35–45% decreased risk in HFrEF |
| Mineralocorticoid receptor antagonists34 | HFrEF and post-infarction LVD | Consistent use of ACEI, variable use of beta-blockers, minimal CRT and ICDs | 35% decreased risk if on beta-blocker; minimal effect if not on beta-blocker |
| Neprilysin inhibitors41 | HFrEF | Robust use of neurohormonal antagonists; CRT in 7% and ICD in 14% | 20% decreased risk overall, ≈50% decreased risk in patients with baseline ICD |
| CRT19,20,36,38,39 | HFrEF | Consistent use of neurohormonal antagonists, variable use of ICD | ≈50% decreased risk in class II/III patients, but no benefit in class IV patients |
| Drugs and devices that suppress or treat ventricular tachyarrhythmias | |||
| ICD2,9,13,19,20 | HFrEF | Consistent use of neurohormonal antagonists, variable CRT | ≈60–70% decreased risk in class II patients, ≈25–40% decreased risk in Class III patients |
| Membrane-active antiarrhythmic drugs17,18 | HFrEF and post-infarction LVD | Variable use of ACEI and beta-blockers, minimal CRT and ICDs | Increased risk of lethal proarrhythmia |
ACEI, angiotensin converting-enzyme inhibitors; CRT, cardiac resynchronization therapy; HFrEF, heart failure with a reduced ejection fraction; ICD, implantable cardioverter-defibrillator; LVD, left ventricular dysfunction.
Are ventricular tachyarrhythmias unrelated to acute ischaemia a mechanism of sudden death?
Ventricular tachycardia and fibrillation are important terminal arrhythmias in patients with HFrEF, and many sudden deaths can be prevented by an ICD,2,9,10 (Table 1). Although the magnitude of the risk reduction is somewhat smaller in those with a non-ischaemic than an ischaemic cardiomyopathy (40–50% vs. 60–70%), the benefit of an ICD in the former group indicates that ventricular tachyarrhythmias can cause sudden death in the absence of coronary artery disease or an acute occlusion.10,11
The action of ICDs to prevent sudden death leads to a decrease in all-cause mortality if the proportion of tachyarrhythmic events to the total number of deaths is large. If sudden deaths comprise half of the deaths and if ICDs prevent half of sudden deaths, then ICDs should lead to a ≈25% reduction in all-cause mortality.2,9 However, the magnitude of the overall survival benefit is attenuated in those with advancing symptoms or comorbidities, in whom non-tachyarrhythmic events contribute importantly to the total deaths.2,11,12 In contrast, in patients with minimal symptoms or end-organ dysfunction, ICDs decrease all-cause mortality, because of the absence of competing risks for death.2,11–13
Cardiac dilatation and scarring are ideal substrates for the initiation of ventricular tachyarrhythmias that can lead to circulatory collapse.14,15 Fibrosis probably underlies the genesis of most sustained ventricular tachyarrhythmias, whether or not the patient has coronary artery disease.15 But what are the acute triggers for these fibrosis-related arrhythmias? Surges in sympathetic nervous system activity and abrupt electrolyte shifts can precipitate arrhythmic events,16 and membrane-active antiarrhythmic agents and digitalis can provoke lethal proarrhythmias.17,18 Yet, in most patients, a trigger for a fatal tachyarrhythmia in a patient with a vulnerable substrate cannot be identified.
More importantly, 30–70% of the sudden deaths in clinical trials of HFrEF are not prevented by an ICD. The failure of ICDs to prevent abrupt circulatory collapse is particularly characteristic of patients with advancing heart failure.2,13,19 As functional capacity worsens from Class II to III, the proportion of sudden deaths that are preventable by an ICD declines from 60–70% to 25–40% (Table 1). In patients with the most severe symptoms, ICDs have not led to a meaningful decrease in the risk of sudden death.2,19,20 These findings indicate that a large proportion of the sudden deaths in patients with progressive ventricular remodelling are not related to an ICD-responsive ventricular tachyarrhythmia.21 What then is the mechanism of sudden death in these individuals?
Is acute mechanical failure a mechanism of sudden death?
Electrocardiographic monitoring during episodes of sudden death has shown that many patients with HFrEF die abruptly without a ventricular tachyarrhythmia. In 40–60% of cardiac arrests, the electrocardiogram exhibits electromechanical dissociation, asystole, or a terminal bradyarrhythmia immediately preceding and at the time of demise.22,23 These findings may be particularly common in patients with a non-ischaemic cardiomyopathy, especially those with advancing symptoms22,23; these are precisely the patients least likely to show a reduced risk of sudden death with an ICD.2,10,11,20 These terminal events are not prevented by electrical devices; they reflect an abrupt cardiac mechanical event, which is the proximate cause for the cardiac arrest.
Interestingly, acute contractile failure may also be the underlying mechanism of sudden death, even if the electrocardiogram manifests ventricular tachycardia or fibrillation. In some patients with an ICD, the ventricular tachyarrhythmia recurs immediately or persists despite repetitive discharges.21 Acute mechanical failure is responsible for the abrupt cessation of circulatory support; the observed tachyarrhythmia represents an epiphenomenon. These events present clinically as sudden deaths that are not preventable by an ICD.
What mechanism can cause a dilated and scarred heart to abruptly stop its mechanical support of the circulation? Progressive fibrosis could conceivably lead to conduction system abnormalities and heart block,15 and acute cardiac distension could theoretically trigger autonomic reflexes,14 resulting in abrupt profound hypotension. However, in most sudden deaths that are not prevented by ICD shocks, there is no identifiable trigger for acute mechanical failure.
The concepts of self-organizing criticality and cascading failure
The process of remodelling is characterized by the slow loss of cardiomyocytes, progressive stretch on the walls of the ventricular chamber and the gradual accumulation of myocardial fibrosis. Since physicians do not expect such a slow incremental process to end abruptly, they have long assumed that sudden death requires a triggering mechanism.
Yet, work in theoretical physics over the past three decades has concluded that slowly progressive processes can (and typically do) end suddenly in the absence of an acute precipitating event. The slow accumulation of snow on a cliff eventually culminates in an avalanche when one incremental snowflake destabilizes the entire structure. Similarly, failing grains of sand lead to the formation of a cone until the slope exceeds a threshold value, at which time one additional grain causes an entire side of the sandcone to collapse. The abrupt loss of structural integrity result from continuation of the same process; it does not require any new trigger, even though the collapse is acute. This framework was first introduced by Bak, Tang, and Wiesenfeld in a famed 1987 paper in Physical Review Letters, which defined the mathematical underpinning of dynamical systems that display ‘self-organizing criticality’.24
Self-organizing criticality is a property of complex systems in which small events trigger major cataclysms due to subtle interdependencies between elements. The ongoing causal process (i.e. falling sand grains or snowflakes) is tolerated for long periods because tiny internal mechanisms ‘self-organize’ in a highly interdependent manner to support overall structural integrity.24,25 However, the ability of the self-organizing process to maintain stability is limited. Once the limit is breeched, the extreme interdependence leads to a ‘cascading failure’, where the tiny fault of one part immediately triggers the failure of other components. When the first part fails, other elements that would normally compensate for the failed component are unduly stressed; the resulting overload causes these to collapse as well, prompting a rapidly evolving cascade of failure.25
This framework—where an abrupt event results from the continuation of tiny increments of the same underlying process rather than a new precipitating mechanism—has been applied to understanding the genesis of avalanches, the sudden appearance of nodes of traffic congestion, and the abrupt onset of system-wide failure of electrical grids. The concept of self-organizing criticality has also recently been used to understand biomedical events, such as protein–protein interactions, cancer, neurodegenerative disease, and genetic and metabolic cascades,26,27 as well as the initiation of cellular and organismal death.
Self-organizing criticality and cascading failure in the remodelled ventricle
The process of cardiac remodelling in patients with HFrEF represents a self-organizing system of highly vulnerable interdependence. ‘Self-organizing criticality’ within the ventricular myocardium relies on complex adaptations to progressive cardiomyocyte stress and stretch, which can come to an abrupt end (‘cascading failure’), thus leading to acute circulatory collapse (i.e. sudden death) in the absence of a new triggering event. Cascading failure in a severely remodelled heart can become manifest either electrically (i.e. ventricular tachyarrhythmia) or mechanically (i.e. asystole, bradyarrhythmia, or electromechanical dissociation).23,28 Incessant ventricular tachyarrhythmias that persist despite repetitive ICD discharges also reflect cascading mechanical failure.21 Because these rhythms are dissociated from mechanical activity, the lethal consequences of mechanical failure cannot be prevented by an ICD.
Is it possible to demonstrate that adverse ventricular remodelling per se leads to cascading acute mechanical failure and sudden death? Cardiac fibrosis and deleterious changes in geometry are related to the activation of endogenous neurohormonal systems (norepinephrine, angiotensin II, aldosterone, and neprilysin), and inhibition of these mechanisms minimizes the development of interdependent critical microsubstrates that can be easily destabilized. Such an effect might explain the ability of each of a broad range of neurohormonal antagonists to reduce the risk of sudden death by 20–40% in patients with post-infarction left ventricular systolic dysfunction or HFrEF (Table 1).29–34 Interestingly, the effect of beta-blockers to reduce the risk of sudden death has been greater than that of angiotensin converting-enzyme inhibitors, possibly because of their more pronounced effect on cardiac remodelling.33 The incremental benefit of mineralocorticoid receptor antagonists on sudden death may be related to their antifibrotic effect and is particularly notable in patients receiving beta-blockers.34
Yet, it is always possible to explain any benefit of neurohormonal antagonists to prevent sudden death to actions of these drugs that are independent of their effects on ventricular remodelling or fibrosis (Take home figure). For example, angiotensin converting-enzyme inhibitors and beta-blockers might prevent a new myocardial infarction that can trigger sudden death.30 Beta-blockers and spironolactone may protect against circadian catecholamine surges and/or electrolyte imbalances that can trigger lethal ventricular tachyarrhythmias.16 These possibilities are difficult to dismiss, since most of the large-scale trials with neurohormonal antagonists were carried out in an era when the background utilization of ICDs was very low, thus making it impossible to distinguish between purely electrical (ICD-preventable) and primarily mechanical (ICD-non-preventable) pathways leading to sudden death. Given these uncertainties—despite compelling evidence linking cardiac remodelling and sudden death35—it has been difficult to confidently ascribe the favourable effects of neurohormonal antagonists on cardiac arrest primarily to the reversal of remodelling-related self-organized criticality.
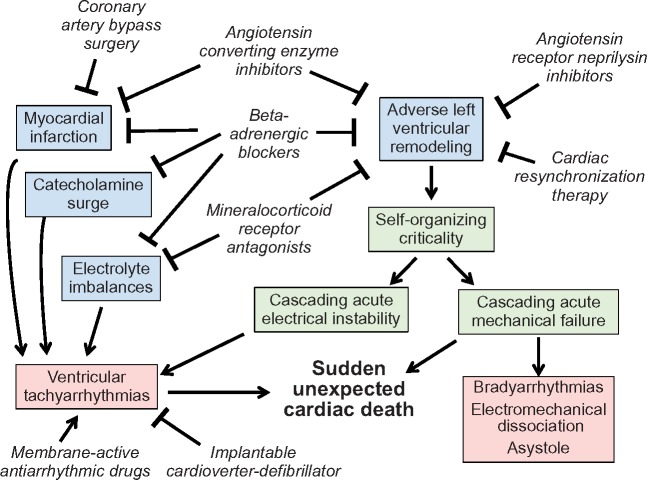
Take home figure.
Mechanisms by which drug, device and surgical interventions reduce the risk of sudden unexpected cardiac death in chronic heart failure. In the absence of an acute precipitating event, adverse left ventricular remodelling and fibrosis generates a substrate of self-organizing criticality, which predisposes to abrupt electrical or mechanical cascading failure. The former leads to sustained ventricular tachycardia or fibrillation, which is often responsive to an implantable cardioverter-defibrillator. In contrast, the latter is manifest by bradyarrhythmias, electromechanical dissociation and asystole and is not responsive to ICD shocks. Ventricular tachycardia or fibrillation that is refractory to repetitive implantable cardioverter-defibrillator shocks is also likely related to acute mechanical failure.
Reversal of remodelling to prevent acute cascading mechanical failure
Fortunately, recent experiences with cardiac resynchronization and neprilysin inhibition in large-scale trials in HFrEF have provided unique opportunities to clarify this confusion. These observations have supported the premise that ventricular remodelling is a direct cause of cascading collapse and sudden death.
First, cardiac resynchronization exerts striking benefits on ventricular remodelling without interfering with neurohormonal systems36; as a result, trials of cardiac resynchronization are poised to identify a unique role for ventricular geometry in the genesis of sudden death. It is therefore noteworthy that, when cardiac resynchronization induces significant reverse remodelling, the substrate for acute electrical cascade is reduced (Take home figure)37,38; the risk of sudden death is decreased by ≈50% in patients without an ICD, primarily related to a decrease in lethal ventricular tachyarrhythmias in those who experience a marked decrease in ventricular volumes (Table 1).38,39 In contrast, in patients with severe symptoms and persistently dilated and fibrotic hearts, a significant risk for sudden death remains following cardiac resynchronization, and it is not reduced by an ICD.19,20
Second, a linkage between ventricular remodelling and acute mechanical failure resulting in sudden death has been supported by studies of sacubitril-valsartan in HFrEF. Neprilysin inhibition has favourable effects on cardiac remodelling and may thereby reduce the substrate for ventricular tachyarrhythmias (Take home figure).40 However, the effect of sacubitril/valsartan to reduce the risk of sudden cardiac death appears to be most marked (>50% risk reduction) in patients who already have an ICD prior to treatment (Table 1).41 The observation that the effect of neprilysin inhibition on sudden death is additive to that of an ICD indicates that the drug influences the risk of cardiac arrest by a mechanism other than (or in addition to) the minimization of ICD-preventable sudden deaths—presumably related to an effect to cause reverse remodelling, and thereby, reduce the risk of acute mechanical failure.
A collective benefit of neurohormonal antagonists and cardiac resynchronization on cardiac remodelling and fibrosis, and thereby, on acute cascading electrical and mechanical failure may explain why the incidence of sudden death in HFrEF has declined over the 10–15 years, in parallel with a reduction in left ventricular cavity size.42,43 This decline has occurred independent of the use of ICDs,42 but coincident with an increase in the utilization of neurohormonal antagonists and cardiac resynchronization therapy. It is noteworthy that the value of ICDs in preventing death in HFrEF was primarily demonstrated at a time when our efforts to minimize ventricular remodelling were not as robust as in the current era.
Summary and conclusions
Sudden death is an important mode of demise in patients with HFrEF. Unaware of this risk, many practitioners often assume that clinically stable patients with mild-to-moderate symptoms do not require intensive therapy. As a result, interventions that can prevent sudden death in HFrEF are underutilized.44
Several pathophysiological mechanisms (e.g. coronary thrombotic ischaemic event, hormone-electrolyte imbalances) can trigger sudden death, but most commonly, cardiac arrest results from acute electrical or mechanical failure in remodelled and fibrotic ventricle. These events typically have no acute precipitant, but the anti-remodelling and antifibrotic effects of neurohormonal antagonists and cardiac resynchronization can prevent sudden death, whether or not an ICD is in place.41 The severely remodelled left ventricle represents a fragile interdependent substrate of self-organized criticality, which can (without warning) lead to acute cascading collapse. This conceptual framework—borrowed from theoretical physics24,25—suggests that life-prolonging treatments in chronic heart failure may have their most important impact if they are applied early in the disease process when ventricular remodelling may be most reversible and when sudden death constitutes a disproportionate number of all deaths.
Conflict of interest: M.P. has recently consulted for Abbvie, Actavis, Akcea, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Gilead, Johnson & Johnson, NovoNordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance.
References
- 1. Packer M. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation 1985;72:681–685. [DOI] [PubMed] [Google Scholar]
- 2. Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, Rogers JG, Anderson J, Johnson GW, Walsh MN, Poole JE, Mark DB, Lee KL, Bardy GH.. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation 2009;120:2170–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L.. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation 2000;102:611–616. [DOI] [PubMed] [Google Scholar]
- 4. Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 5. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G; Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1231–1239. [DOI] [PubMed] [Google Scholar]
- 6. Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B; COMMANDER HF Investigators. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 2018;379:1332–1342. [DOI] [PubMed] [Google Scholar]
- 7. Cleland JG, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, Prentice C, Ford I, Trainer A, Poole-Wilson PA.. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J 2004;148:157–164. [DOI] [PubMed] [Google Scholar]
- 8. Carson P, Wertheimer J, Miller A, O'Connor CM, Pina IL, Selzman C, Sueta C, She L, Greene D, Lee KL, Jones RH, Velazquez EJ; STICH Investigators. The STICH trial: mode-of-death results. JACC Heart Fail 2013;1:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML; MADIT-II Investigators. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol 2004;43:1459–1465. [DOI] [PubMed] [Google Scholar]
- 10. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S; DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 11. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 12. Steinberg BA, Al-Khatib SM, Edwards R, Han J, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, Dorian P, Hallstrom A, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Inoue LY, Sanders GD.. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail 2014;2:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedman DJ, Al-Khatib SM, Zeitler EP, Han J, Bardy GH, Poole JE, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, Dorian P, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Inoue LYT, Sanders GD.. New York Heart Association class and the survival benefit from primary prevention implantable cardioverter defibrillators: a pooled analysis of 4 randomized controlled trials. Am Heart J 2017;191:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen DE, Craig CS, Hondeghem LM.. Stretch-induced arrhythmias in the isolated canine ventricle. Evidence for the importance of mechanoelectrical feedback. Circulation 1990;81:1094–1105. [DOI] [PubMed] [Google Scholar]
- 15. Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ.. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol 2011;57:821–828. [DOI] [PubMed] [Google Scholar]
- 16. Packer M, Gottlieb SS, Kessler PD.. Hormone-electrolyte interactions in the pathogenesis of lethal cardiac arrhythmias in patients with congestive heart failure. Basis of a new physiologic approach to control of arrhythmia. Am J Med 1986;80:23–29. [DOI] [PubMed] [Google Scholar]
- 17. Hohnloser SH, Halperin JL, Camm AJ, Gao P, Radzik D, Connolly SJ; PALLAS investigators. Interaction between digoxin and dronedarone in the PALLAS trial. Circ Arrhythm Electrophysiol 2014;7:1019–1025. [DOI] [PubMed] [Google Scholar]
- 18. Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP.. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Lancet 1996;348:7–12. [DOI] [PubMed] [Google Scholar]
- 19. Lindenfeld J, Feldman AM, Saxon L, Boehmer J, Carson P, Ghali JK, Anand I, Singh S, Steinberg JS, Jaski B, DeMarco T, Mann D, Yong P, Galle E, Ecklund F, Bristow M.. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation 2007;115:204–212. [DOI] [PubMed] [Google Scholar]
- 20. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; COMPANION Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 21. Pires LA, Lehmann MH, Steinman RT, Baga JJ, Schuger CD.. Sudden death in implantable cardioverter-defibrillator recipients: clinical context, arrhythmic events and device responses. J Am Coll Cardiol 1999;33:24–32. [DOI] [PubMed] [Google Scholar]
- 22. Faggiano P, d’Aloia A, Gualeni A, Gardini A, Giordano A.. Mechanisms and immediate outcome of in-hospital cardiac arrest in patients with advanced heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2001;87:655–657, A10-1. [DOI] [PubMed] [Google Scholar]
- 23. Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J.. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 1989;80:1675–1680. [DOI] [PubMed] [Google Scholar]
- 24. Bak P, Tang C, Wiesenfeld K.. Self-organized criticality: an explanation of 1/ƒ noise. Phys Rev Lett 1987;59:381–384. [DOI] [PubMed] [Google Scholar]
- 25. Bak P, Tang C, Wiesenfeld K.. Self-organizing criticality. Phys Rev A 1988;38:364–374. [DOI] [PubMed] [Google Scholar]
- 26. Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, Petersen RC, Weiner MW, Jack CR Jr; Alzheimer’s Disease Neuroimaging Initiative. Cascading network failure across the Alzheimer's disease spectrum. Brain 2016;139:547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun L, Wang S, Li K, Meng D.. Analysis of cascading failure in gene networks. Front Genet 2012;3:292.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamori S, Ismail H, Ngo LH, Manning WJ, Nezafat R.. Left ventricular geometry predicts ventricular tachyarrhythmia in patients with left ventricular systolic dysfunction: a comprehensive cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2017;19:79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garg R, Yusuf S; Collaborative Group on ACE Inhibitor Trials. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450–1456. [PubMed] [Google Scholar]
- 30. Domanski MJ, Exner DV, Borkowf CB, Geller NL, Rosenberg Y, Pfeffer MA.. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 1999;33:598–604. [DOI] [PubMed] [Google Scholar]
- 31. Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL.. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106:2194–2199. [DOI] [PubMed] [Google Scholar]
- 32. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 33. Krum H, van Veldhuisen DJ, Funck-Brentano C, Vanoli E, Silke B, Erdmann E, Follath F, Ponikowski P, Goulder M, Meyer W, Lechat P, Willenheimer R; behalf of the CIBIS III Investigators. Effect on mode of death of heart failure treatment started with bisoprolol followed by Enalapril, compared to the opposite order: results of the randomized CIBIS III trial. Cardiovasc Ther 2011;29:89–98. [DOI] [PubMed] [Google Scholar]
- 34. Rossello X, Ariti C, Pocock SJ, Ferreira JP, Girerd N, McMurray JJV, Van Veldhuisen DJ, Pitt B, Zannad F.. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left-ventricular systolic dysfunction: an individual patient-level meta-analysis of three randomized-controlled trials. Clin Res Cardiol 2019;108:477–486. [DOI] [PubMed] [Google Scholar]
- 35. Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G.. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol 2011;57:1468–1476. [DOI] [PubMed] [Google Scholar]
- 36. Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, Hall WJ, Pfeffer MA, Moss AJ; MADIT-CRT Investigators. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchron-ization therapy. Circulation 2010;122:985–992. [DOI] [PubMed] [Google Scholar]
- 37. Adelstein EC, Schwartzman D, Jain S, Bazaz R, Wang NC, Saba S.. Left ventricular dimensions predict risk of appropriate shocks but not mortality in cardiac resynchronization therapy-defibrillator recipients with left bundle-branch block and non-ischemic cardiomyopathy. Europace 2017;19:1689–1694. [DOI] [PubMed] [Google Scholar]
- 38. Barsheshet A, Wang PJ, Moss AJ, Solomon SD, Al-Ahmad A, McNitt S, Foster E, Huang DT, Klein HU, Zareba W, Eldar M, Goldenberg I.. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT. J Am Coll Cardiol 2011;57:2416–2423. [DOI] [PubMed] [Google Scholar]
- 39. Uretsky BF, Thygesen K, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cleland JG.. Predictors of mortality from pump failure and sudden cardiac death in patients with systolic heart failure and left ventricular dyssynchrony: results of the CARE-HF trial. J Card Fail 2008;14:670–675. [DOI] [PubMed] [Google Scholar]
- 40. Martens P, Nuyens D, Rivero-Ayerza M, Van Herendael H, Vercammen J, Ceyssens W, Luwel E, Dupont M, Mullens W.. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol 2019;doi:10.1007/s00392-019-01440-y. [DOI] [PubMed] [Google Scholar]
- 41. Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD.. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J 2015;36:1990–1997. [DOI] [PubMed] [Google Scholar]
- 42. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Køber L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray J.. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 43. Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, Fox KA, Rajwani A, Baig W, Groves D, Barlow P, Fisher AC, Batin PD, Kahn MB, Zaman AG, Shah AM, Byrne JA, Lindsay SJ, Sapsford RJ, Wheatcroft SB, Witte KK, Kearney MT.. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail 2011;4:396–403. [DOI] [PubMed] [Google Scholar]
- 44. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]