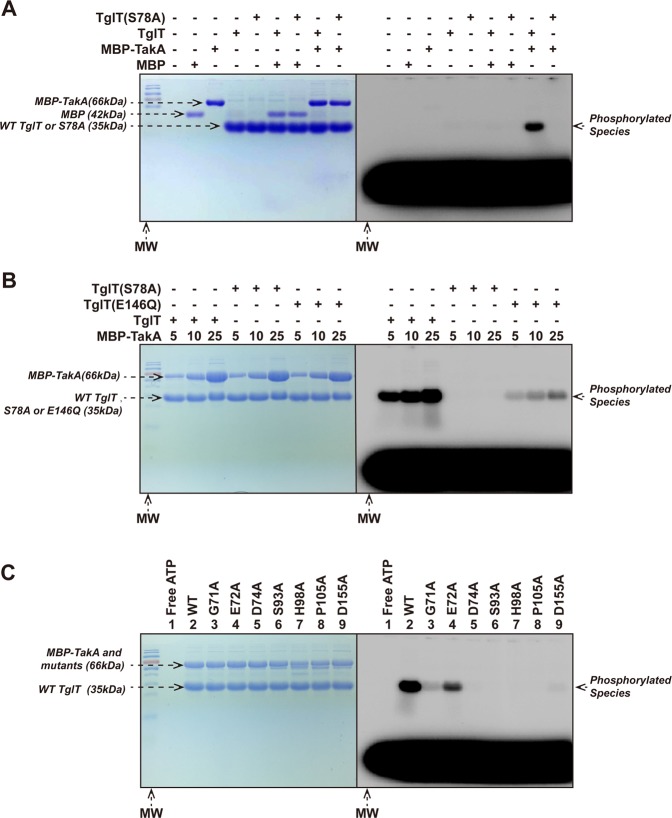
Fig. 5. In vitro kinase assay TakA directly phosphorylates TglT in vitro.
In each kinase reaction, radioactive probe [γ-32P] ATP was included to visualize the phosphorylated protein species. An excess of unlabeled ATP (6×104 folds over the labeled ATP) was added to remove the non-covalently bound probe. The indicated kinase reactions were resolved by SDS-PAGE. The SDS-PAGE was first exposed to phosphoscreen and visualized with Typhoon imager (right); the same SDS-PAGE was then stained with Coomassie brilliant blue (left). a Purified, non-phosphorylated TglT and the S78A mutant were incubated with MBP-TakA or MBP (as nonspecific control). In the presence of MBP-TakA, phosphorylated TglT was detected, whereas TglT S78A was not phosphorylated. b Mutation S78A of TglT prevented TakA catalyzed phosphorylation, whereas E146Q impaired the phosphorylation. As the concentration of TakA increased, more phosphorylated TglT E146Q was observed, but the phosphorylation efficiency was lower than wildtype TglT. c Conserved residues of TakA were critical to its kinase activity. wildtype TglT was incubated with MBP-TakA (lane 2) and annotated TakA mutants including: G71A (lane 3), E72A (lane 4), D74A (lane 5), S93A (lane 6), H98A (lane 7), P105A (lane 8) and D155A (lane 9); lane 1: a free ATP control. While E72A retained similar activity as the wildtype protein, the other mutants showed loss of the activity to different extents. Especially, D74A, S93A, H98A, P105A and D155A showed complete loss of kinase activity. The conserved residues of TakA were identified by structure-based multiple sequence alignment, see Supplementary Fig. 13. Source data are provided as a Supplementary Data 2.