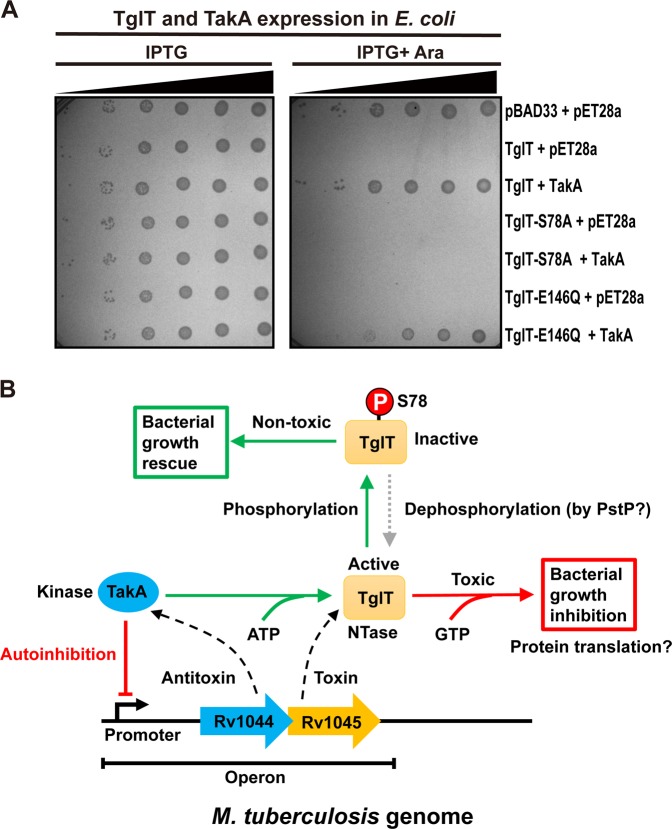
Fig. 6. Phosphorylation of TglT results in toxicity neutralization and a proposed model of the Rv1044-Rv1045 TA system.
a The antitoxicity mechanism of TakA involves phosphorylation of TglT at S78 site. BL21 cells expressing annotated proteins were spotted on M9 plates with 10-fold serial dilutions from the right to the left: 10−1 10−2 10−3, 10−4, 10−5 and 10−6, respectively. The plate on the left contained IPTG inducing TakA expression, whereas the plate on the right contained both IPTG and L-arabinose inducing toxin and antitoxin expressions. Bacterial growth was examined after overnight incubation. The toxicity of S78A was not rescued by TakA because the mutant cannot be phosphorylated. The neutralization of TglT E146Q toxicity was about 10-fold less efficient that that of wildtype TglT, because the phosphorylation of TglT E146Q by TakA was also less efficient (Fig. 5b). b The Rv1044 gene (blue arrow) encodes the atypical serine protein kinase TakA (blue ellipse), acting as the antitoxin, while the Rv1045 gene (orange arrow) encodes the guanylyltransferase TglT (orange rectangle), acting as the toxin. TakA negatively regulates its own promoter. TglT binds GTP and targets a vital cellular process, which leads to bacterial growth arrest. A possible cellular process targeted by TglT is protein translation. TakA recognizes TglT and phosphorylates the S78 residue, thus inhibiting the catalytic activity of TglT resulting in the neutralization of toxicity. The gray dashed arrow indicates the reversal of antitoxicity, which required an unknown phosphatase. The PstP phosphatase encoded by Mtb is a possible candidate. Source data are provided as a Supplementary Data 2.