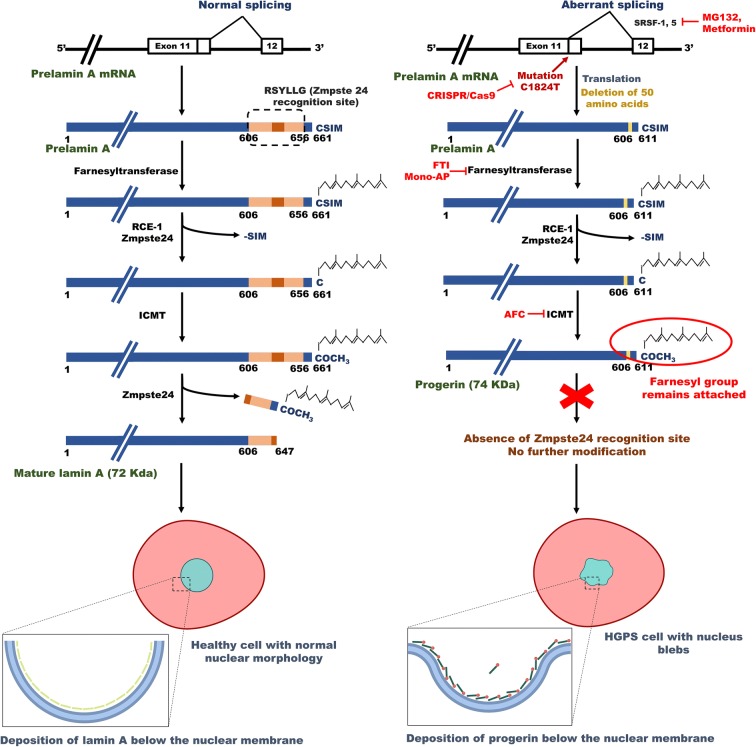
Fig. 1.
Biogenesis of lamin A and progerin in the cell. The left picture depicts the expression of normal lamin A protein from the LMNA gene. The normal prelamin A protein undergoes extensive modifications like farnesylation and carboxymethylation at the C-terminal. Finally, the C-terminal is removed by the activity of Zmpste24 endopeptidase to produce mature lamin A. Therefore, the mature lamin A protein does not contain the farnesyl group. The right picture shows the formation of progerin from mutant LMNA gene. Mutation in LMNA gene in exon 11 (1824 C>T) leads to generation of an aberrant splicing site. Splicing at this abnormal site causes deletion of 50 amino acids (607–656) in the prelamin A protein. As a result, prelamin A Δ50 loses the Zmpste24-recognition site, which causes the retention of modified C-terminal with the farnesyl group. This protein is now called as progerin. Accumulation of progerin at nuclear lamina leads to defects in nuclear morphology and function. Various therapeutic interventions may interfere with the biogenesis of progerin. The CRISPR–Cas9 system can correct the causative mutation, whereas MG132 and metformin can inhibit the aberrant splicing. FTIs (farnesyl transferase inhibitors) and mono-AP (mono-aminopyrimidines) can thwart farnesylation of progerin and AFC (N-acetyl-S-farnesyl-l-cysteine) inhibits methylation of the C-terminal of progerin. Abbreviations: ICMT, isoprenylcysteine carboxyl methyltransferase; RCE-1, Ras-converting enzyme; SRSF-1 and -5, serine/arginine-rich splicing factor-1 and -5