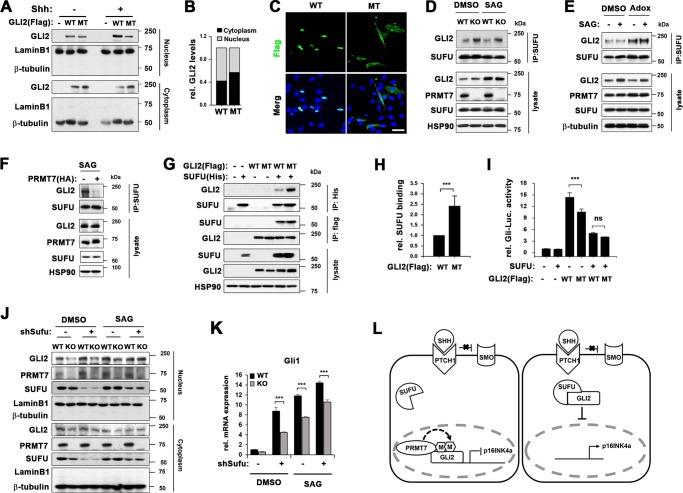
Fig. 7.
PRMT7 negatively regulates GLI2 binding to SUFU. a Cellular fractionation of wildtype GLI2 (WT) or GLI2 R225/227 K mutant (MT), which were treated with Shh for 24 h after 16 h starvation. b Quantification of cytoplasmic or nucleic WT or MT shown in panel a. c Confocal microscopy for Flag-GLI2 in 10T1/2 cells transfected with WT or MT. Scale bar, 50 μm. d Coimmunoprecipitation with anti-SUFU antibody in WT and KO MEFs, which were treated with DMSO or SAG for 8 h, followed by immunoblotting with anti-GLI2 antibody. e Coimmunoprecipitation with anti-SUFU antibody in 10T1/2 cells, which were pretreated with DMSO or Adox prior to SAG treatment for 8 h, followed by immunoblotting with anti-GLI2 antibody. f Coimmunoprecpitation with anti-SUFU antibody in control-expressing or PRMT7-expressing 10T1/2 cells, which were treated with SAG for 8 h, followed by immunoblotting with anti-GLI2 antibody. g Immunoprecipitation analysis with anti-His antibody or anti-Flag antibody in 293 T cells, which were transfected with Flag-tagged WT or MT, and/or His-tagged SUFU, followed by immunoblotting with anti-Flag antibody or anti-His antibody, respectively. h Quantification for the relative SUFU binding of WT or MT shown in panel h. The results are shown as mean ± SD of triplicate experiments. ***P < 0.001. i Gli-reporter assay in 10T1/2 cells transfected with SUFU, and WT or MT as indicated. Data represent means of triplicates ± SD. n = 3, **P < 0.01, ***P < 0.001. j Cellular fractionation of WT or KO MEFs infected with lentivirus expressing control or SUFU shRNA and 36 h later, treated with DMSO or SAG for 8 h. k qRT-PCR for Gli1 expression in the replica sets shown in panel k. Data represent mean ± SD. n = 3, ANOVA test for comparison, ***P < 0.001. l A working hypothesis of the mechanism by which PRMT7 regulates GLI2 activity. PRMT7 methylates GLI2 at R225/227 and interferes with GLI2 binding to SUFU thereby activating GLI2 mediated inhibition of p16 and cellular senescence