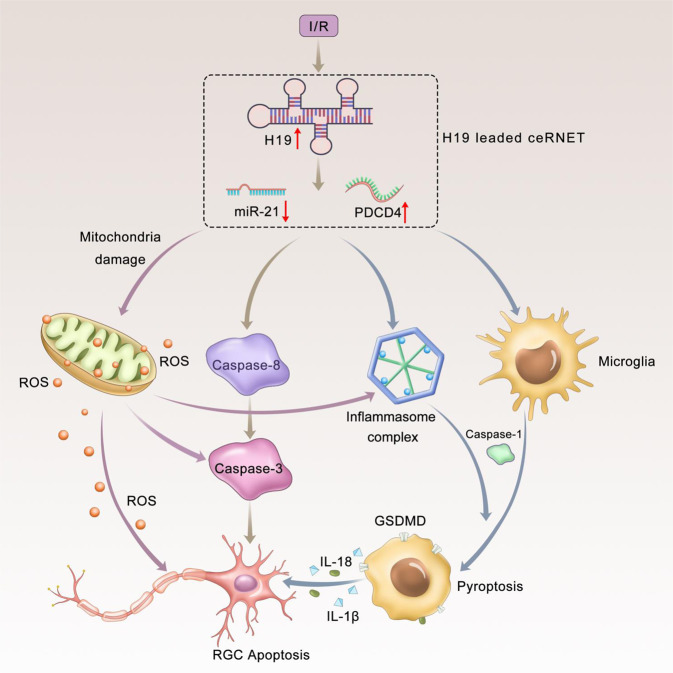
Fig. 6.
Graphical summary of H19/miR-21/PDCD4 ceRNET in I/R injury. H19 responded to ischemic insults with increased expression level. Upregulated H19 then competed with PDCD4 for miR-21 to form the ceRNET. The ceRNET is capable of processing several pathological signals into a unitary and coherent adaptive response. H19-mediated ceRNET triggered reciprocal activation of NLRP3/NLRP6 inflammasomes. Thereafter, activated caspase-1 was recruited to cleave GSDMD, which characterized microglial pyroptosis. Meanwhile, on the inflammasome platform, mature forms of IL-1β and IL-18 were managed, which mediated pyroptosis to exert neurotoxicity. Besides inflammatory caspases, the H19/miR-21/PDCD4 ceRNET also activated apoptotic caspases (caspase-3 and caspase-8) to directly regulate RGC apoptosis in I/R injury. Mitochondrial dysfunction (marked by MMP collapse) and consequent ROS overproduction were identified as independent activators of inflammasomes and apoptotic signals. H19 knockout effectively relieved I/R-induced neuro-inflammation, neuronal death and mitochondrial failure. Simultaneous regulation of the H19/miR-21/PDCD4 ceRNET further blocked the ischemic cascade to improve the protective effect. I/R, ischemia/reperfusion injury; RGC, retinal ganglion cell; MMP, mitochondrial membrane potential; ROS, reactive oxygen species