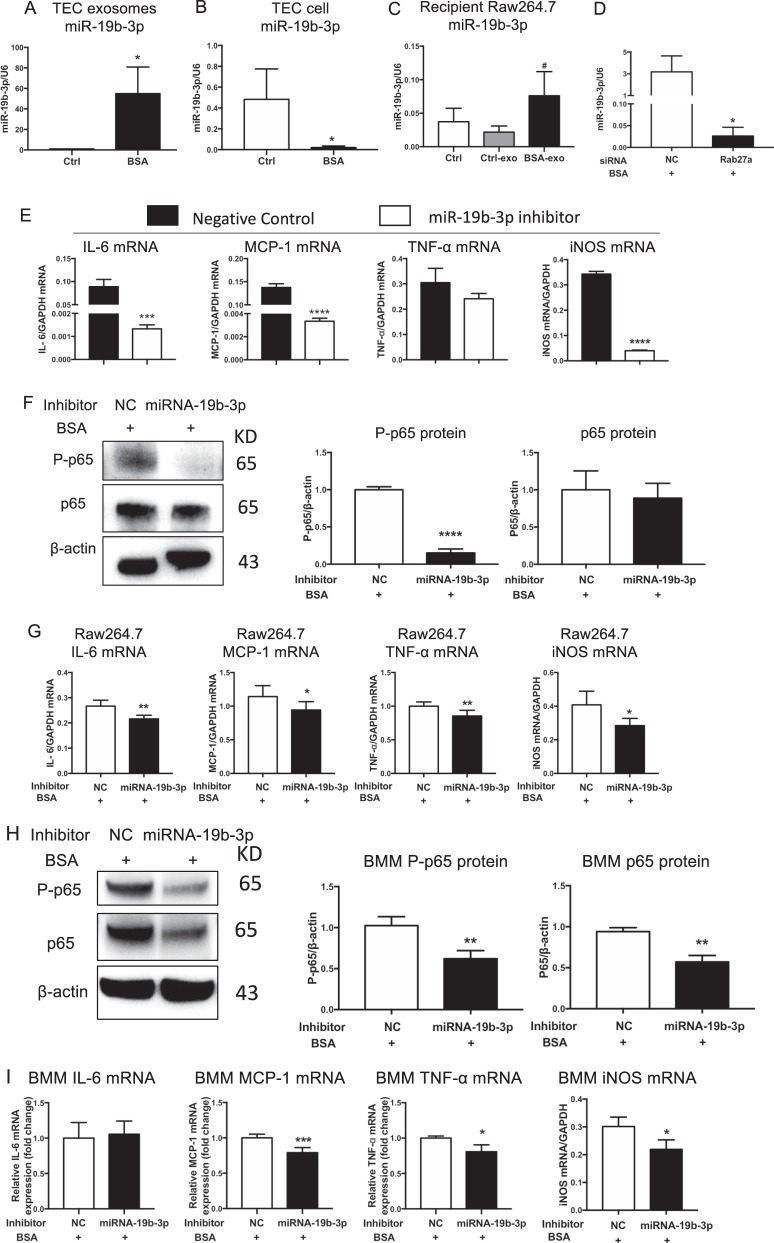
Fig. 4.
TECs exosomes promoted M1 macrophage activation via miR-19b-3p. a–c Expression of miR-19b-3p in TEC-derived exosomes (*p = 0.0225 vs Ctrl TEC-derived exosomes), TEC cells (*p = 0.0491 vs Ctrl TECs) and recipient Raw264.7 macrophages treated with TEC-derived exosomes (#p = 0.0495 vs Ctrl Raw264.7 macrophages), respectively. d Expression of miR-19b-3p in TEC-derived exosomes with Rab27a silencing. *p = 0.0269 vs NC. e Inflammatory cytokines and iNOS expression in macrophages treated with exosomes from TECs transfected with miR-19b-3p inhibitor or NC. Exosomes were purified and applied to recipient Raw264.7 macrophages. Upregulation of IL6, MCP-1 and iNOS mRNA was remarkably reversed in TEC-exo with miR-19b-3p inhibitor. ***p = 0.0007; ****p < 0.0001 vs cells with NC inhibitor. f, g Raw264.7 macrophages were transfected with NC or miR-19b-3p inhibitor in the presence of BSA. f Representative western blotting of three independent experiments, and quantification of P-p65(****p < 0.0001 versus NC) and p65 in Raw264.7 macrophages. g Inflammatory cytokine (IL6, **p = 0.0025; MCP-1, *p = 0.0463; TNF- α, **p = 0.0072 vs NC) and iNOS mRNA expression (**p = 0.0299 vs NC) in Raw264.7 macrophages was detected by RT-PCR. h, i Bone marrow-derived macrophages (BMMs) were transfected with NC or miR-19b-3p inhibitor in the presence of BSA. h Representative western blotting of three independent experiments, and quantification of P-p65(**p = 0.0089 versus BMMs with NC inhibitor), p65(**p = 0.0023 versus BMMs with NC inhibitor) in BMMs after transfection. I Inflammatory cytokine (IL6; MCP-1, ***p = 0.00045; TNF- α, p = 0.0277 vs NC) and iNOS (*p = 0.014 vs NC) mRNA expression in BMMs was detected by RT-PCR. NC negative control, Ctrl exo exosomes from TECs without BSA, BSA-exo exosomes from TECs with BSA treatment. Data presented as mean ± S.E.M. of three independent experiments. p values were calculated using unpaired Student’s t-test