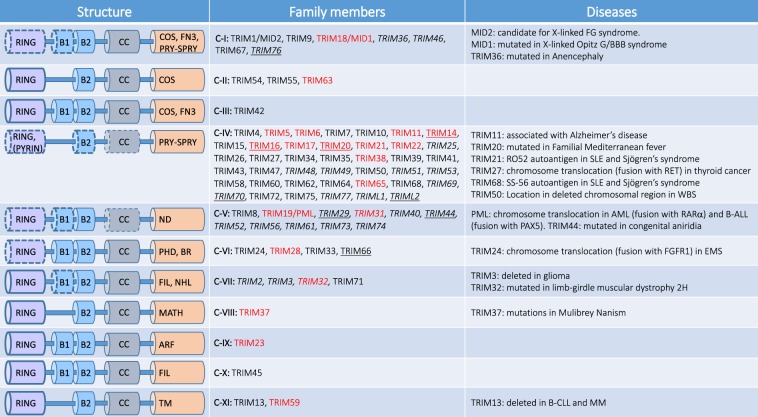
Fig. 1. Classification of TRIM proteins.
TRIM proteins are characterized by the presence of a tripartite domain consisting of a RING finger, two B-boxes (B1 and B2) and a coiled-coil domain. TRIM proteins are divided into 11 subclasses (from C-I to C-XI) on the basis on their C-terminal domain. C-terminal domains expressed by TRIM subgroups are: ARF ADP-ribosylation factor-like, BR bromodomain, COS C-terminal subgroup one signature, FIL filamin-type immunoglobulin, NHL NHL domain, FN3 fibronectin type 3, PRY-SPRY PRY-SPRY domain, MATH meprin and tumor-necrosis factor receptor-associated factor homology, PHD plant homeodomain, TM transmembrane. Some members of TRIM subclasses lack the RING domain (underlined), B-boxes or Coiled-Coil (CC) domains (italics), ND: TRIM lacking a unique C-terminal domain. As indicated in the main text, members of the C-VI constitute the TIF1 family of chromatin binding proteins. TRIM proteins that play a role in autophagy are indicated in red. FG: X‐linked multiple congenital anomalies syndrome. SLE systemic lupus erythematosus, WBS Williams–Beuren syndrome, AML acute myeloid leukemia, B-ALL B-cell precursor acute lymphoblastic leukemia, EMS 8p11 myeloproliferative syndrome, B-CLL B-cell chronic lymphocytic leukemia, MM multiple myeloma.