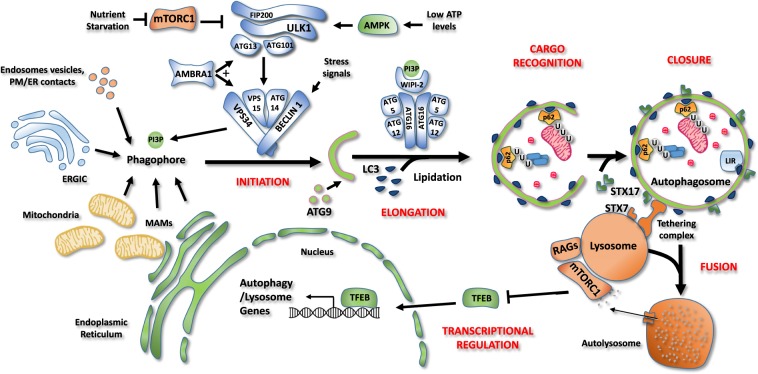
Fig. 2. Schematic representation of the autophagy core machinery.
Description of the different steps of the autophagy process. Initiation. Autophagosome formation is under the control of the ULK1 and BECLIN 1 complexes. Under nutrient-rich conditions, mTORC1 associates with and inhibits ULK1 complex (ATG101, ATG13, FIP200, and ULK1/2). During nutrient starvation, mTORC1 is inhibited, allowing ULK1 complex to induce autophagy. When ATP levels are low, the AMP-activated protein kinase (AMPK) acts as a positive regulator of the ULK1 complex. Once active, ULK1 phosphorylates BECLIN 1 and stimulates the lipid kinase activity of the BECLIN 1 complex (BECLIN 1, ATG14, VPS34, VPS15). This complex is responsible for the production of phosphatidylinositol 3-phosphate (PI3P) by the Class III PI-3-kinase, VPS34. PI3P allows the nucleation of an isolation membrane, also called phagophore, which may originate from endoplasmatic reticulum (ER), plasma membrane, Golgi complex, endosomes vesicles or endoplasmatic reticulum-mitochondrial contact sites (MAMs). Elongation, cargo recognition and closure. Autophagosome expansion and closure requires the fusion of the isolation membrane with ATG9-positive vesicles and the insertion of ATG8 family members (LC3, GABARAP, GATE-16) on autophagosome membranes upon lipidation. WIPI-2 binds PI3P on forming vesicles as well as ATG16, thus allowing the recruitment of both ATG12-5-16 complex, which is necessary for LC3 lipidation. LC3 allows cargo recruitment by interacting with the adaptor proteins, such as the ubiquitin binding protein p62 and proteins containing the LC3-interacting region (LIR) motif. Then, phagophore closes to form a double-membrane vesicle, named autophagosome. Fusion. Finally, autophagosomes are recruited to lysosomes by a tethering complex and fuse through SNARE proteins SINTAXIN 7 (STX79 and SINTAXIN 17 (STX17), forming a single membrane vesicle, named autolysome, where sequestered materials are degraded. Transcriptional regulation. Autophagy process is tightly regulated also at transcriptional level by several transcription factors. Under amino acid sufficiency, mTORC1 inhibits the transcription factor EB (TFEB) causing its retention in the cytosol and blocking its transcriptional activity. Upon nutrient starvation, mTORC1 is inactivated, leading to TFEB nuclear translocation and transcription of genes involved in lysosomal biogenesis and autophagosome formation.