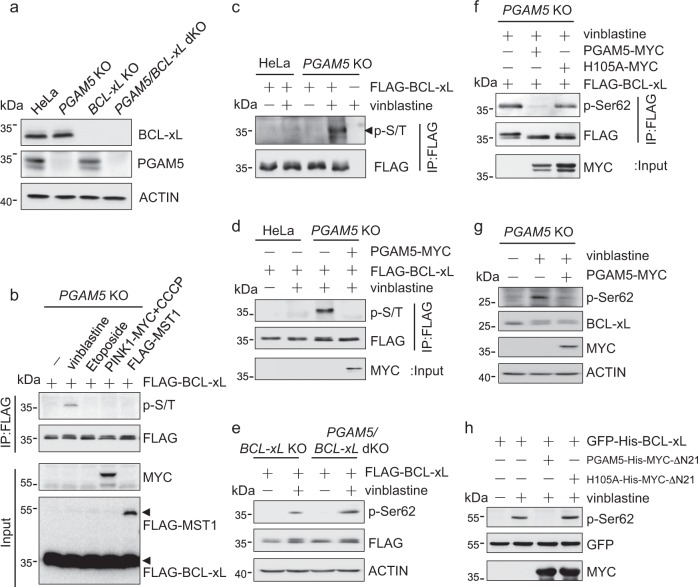
Fig. 1.
The vinblastine-induced phosphorylation of BCL-xL at Ser62 is reversed by the mitochondrial phosphatase PGAM5. a Western blots of lysates from wild-type, PGAM5 knockout, BCL-xL knockout or PGAM5/BCL-xL double knockout HeLa cells using the indicated antibodies. b PGAM5 knockout HeLa cells expressing FLAG-BCL-xL with or without co-expression of PINK1-MYC or FLAG-MST1 were treated with vinblastine (500 nM), Etoposide (50 uM), CCCP (50 μM) or the vehicle for 24 h. The cells were lysed for immunoprecipitation (IP) with anti-FLAG antibody and western blotting with antibodies recognizing the FLAG or MYC epitope or phospho-Ser/Thr (p-S/T). c Wild-type or PGAM5 knockout HeLa cells expressing FLAG-BCL-xL were treated with vinblastine or the vehicle for 24 h, lysed and immunoprecipitated with anti-FLAG antibody. Total and phospho-BCL-xL were detected by western blotting with anti-FLAG or anti-p-S/T antibody, respectively. d Wild-type or PGAM5 knockout HeLa cells expressing PGAM5-MYC and/or FLAG-BCL-xL were treated with vinblastine or the vehicle. The FLAG-BCL-xL in the cell lysate was enriched by IP, and the level of phospho-BCL-xL was determined by western blotting with the p-S/T antibody. e BCL-xL single or PGAM5/BCL-xL double knockout HeLa cells expressing FLAG-BCL-xL were treated with vinblastine or the vehicle for 24 h, lysed and western blotted with the indicated antibodies including one that recognizes the phospho-Serine62 of BCL-xL (p-Ser62). f PGAM5 knockout HeLa cells expressing PGAM5-MYC or the phosphatase-dead H105A mutant, and/or FLAG-BCL-xL were treated with vinblastine, lysed, immunoprecipitated with anti-FLAG antibody, and western blotted with the p-Ser62 antibody to detect the level of BCL-xL phosphorylation at Ser62. g PGAM5 knockout HeLa cells with or without PGAM5-MYC expression were treated with vinblastine and lysed. The endogenous phospho-Ser62-BCL-xL was detected by western blotting using the p-Ser62 antibody. Note that the endogenous BCL-xL in the vinblastine-treated cells was detected as double bands by western blotting using the anti-BCL-xL antibody. Although the nature of the double bands is unknown, it is unlikely due to the Ser62 phosphorylation, which was detected by the anti-phospho-Ser62 antibody as an extra-band above the double bands. h PGAM5/BCL-xL double knockout HeLa cells expressing GFP-His-BCL-xL were treated with vinblastine and lysed. The immunoprecipitated GFP-His-BCL-xL was incubated with purified PGAM5-His-MYC-ΔN21 protein or the phosphatase-dead H105A mutant. The phospho-Ser62-BCL-xL was detected by western blotting using the p-Ser62 antibody