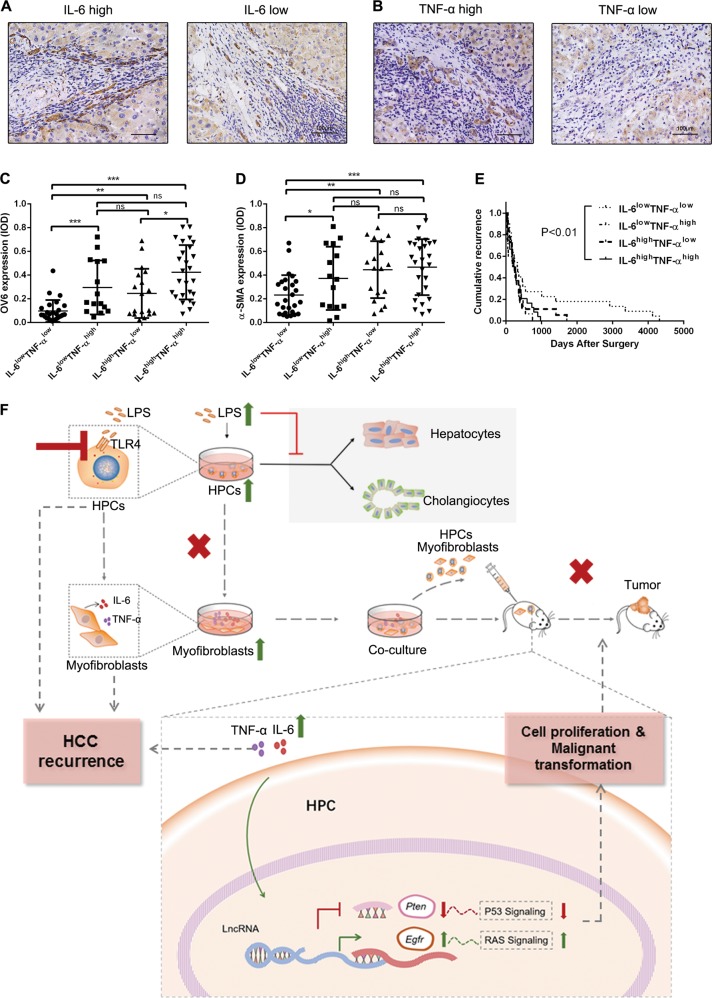
Fig. 7.
Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) level in peritumoral tissue correlated with hepatic progenitor cell (HPC) activation, hepatic fibrosis, and hepatocellular carcinoma (HCC) recurrence. a, b IL-6 and TNF-α expression was detected in HCC peritumoral tissues by immunohistochemical analysis. c, d. OV6 and α-smooth muscle actin (α-SMA) expression was analyzed in each group. *p < 0.05, **p < 0.01, and ***p < 0.001. e. Recurrence rate was analyzed in each group of HCC patients (Kaplan–Meier method); p values were obtained by log-rank multiple comparison tests. f A schematic model of the mechanism underlying the role of lipopolysaccharide (LPS) induced HPC differentiation into myofibroblasts in the malignant transformation of HPCs. LPS induced HPCs to differentiate into myofibroblasts, which led to the proliferation and malignant transformation of HPCs by secretion of IL-6 and TNF-α. The potential mechanism was that LPS/Toll-like receptor 4 (TLR4) signaling induced differentiation of HPCs into myofibroblasts and production of IL-6 and TNF-α. IL-6 and TNF-α promoted the upregulation of epidermal growth factor receptor (EGFR) and downregulation of phosphatase and tensin homolog (PTEN) through long non-coding RNA (lncRNA) regulation, which resulted in the aberrant expression of Ras and p53 signaling pathways in HPCs and finally enhanced the proliferation and malignant transformation of HPCs. ns, not significant