Abstract
The data presented in this paper are related to the research article titled “Redox Behaviour of [Ru(β-diketonato)3] Compounds” [1]. This paper presents structural and energy data obtained from the density functional theory (DFT) computations. The energy data is related to experimentally obtained redox potential values. Various relationships are presented for the RuIII/II and RuIII/IV redox couples, involving both their experimental redox data as well as DFT calculated data, such as frontier orbital energies (EHOMO and ELUMO) and calculated Mulliken electronegativity values.
Keywords: Ruthenium, Acac, DFT, Relationships, HOMO, LUMO, Oxidation, Reduction
Specifications Table
| Subject | Chemistry |
| Specific subject area | Computational chemistry |
| Type of data | Table Graph Figure |
| How data were acquired | Electronic structure calculations, using the Amsterdam Density Functional (ADF) 2018 and Gaussian 16 programmes. |
| Data format | Raw Analyzed |
| Parameters for data collection | Input coordinates were constructed manually, using ChemCraft |
| Description of data collection | Computational DFT data was obtained with the ADF 2018 and Gaussian 16 programmes on the High Performance Computing facility of the University of the Free State |
| Data source location | Department of Chemistry, University of the Free State, Nelson Mandela Street, Bloemfontein, South Africa |
| Data accessibility | Data is included with article and in the supplementary file |
| Related research article | J. Conradie, Redox Behaviour of [Ru(β-diketonato)3] Compounds. Electrochim. Acta. 337 (2020) 135801. https://doi:10.1016/j.electacta.2020.135801. |
Value of the Data
-
•
Density functional theory (DFT) calculated optimized xyz-data (coordinates) for a series of 14 tris(β-diketonato)ruthenium(III) compounds are provided
-
•
DFT optimized geometrical data (coordinates) can be used to visualize the DFT calculated structures of a series of 14 tris(β-diketonato)ruthenium(III) compounds
-
•
This data provides highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies (EHOMO and ELUMO) of different tris(β-diketonato)ruthenium(III) compounds
-
•
Relationships between experimental redox data and DFT calculated frontier orbital energies and calculated Mulliken electronegativity (χ) for tris(β-diketonato)ruthenium(III) compounds containing different electron donating and electron withdrawing substituents, obtained by different DFT methods, all produced similar R2 values
-
•
EHOMO, ELUMO and χcalc data obtained by the different DFT methods show the same trend, namely [Ru(β-diketonato)3] compounds containing electron withdrawing substituents on the β-diketonato ligand have lower EHOMO and ELUMO, and higher χcalc values than [Ru(β-diketonato)3] compounds containing electron donating substituents on the β-diketonato ligand
-
•
Electronic energy data of different spin states of neutral, oxidized and reduced tris(acetylacetonato)ruthenium(III) provide the lowest energy spin state of the neutral, oxidized and reduced tris(acetylacetonato)ruthenium(III)
-
•
Linear relationships obtained from this data enable further prediction of the properties of novel complexes prior to synthesis, to be confirmed by laboratory tests
1. Data Description
This data article provides data related to Ru(III) compounds 1 – 14 (Fig. 1). A summary of the Hammett meta-substituent sigma constants, σR [2], of both the R and Rꞌ substituents on the β-diketonato ligand of the [Ru(β-diketonato)3] compounds 1 – 14, is provided in Table 1. The σR values provide an indication of the electron donating (smaller value) and electron withdrawing (larger value) property of the individual substituents R and Rꞌ on the β-diketonato ligand of the [Ru(β-diketonato)3] compounds 1 – 14. On the other hand, the data of the sum (σR + σR') provides an indication of the electron donating (smaller value) and electron withdrawing (larger value) property of the β-diketonato ligand with its two substituents.
Fig. 1.
Structure of the fourteen [Ru(β-diketonato)3] compounds 1 – 14.
Table 1.
Hammett meta-substituent sigma constants, σR, of the individual R and Rꞌ groups [2] substituted on the β-diketonato ligand of the [Ru(β-diketonato)3] compounds 1 – 14, with the R and Rꞌ substituents as shown in Fig. 1. (σR+ σRꞌ) gives the combined electronic effect of each ligand containing two substituents.
| Compound no | R | R' | σR | σR' | (σR + σR') |
|---|---|---|---|---|---|
| 1 | CF3 | CF3 | 0.43 | 0.43 | 0.86 |
| 2 | CF3 | C4H3O | 0.43 | 0.06 | 0.49 |
| 3 | CF3 | C4H3S | 0.43 | 0.09 | 0.52 |
| 4 | CF3 | Ph | 0.43 | 0.06 | 0.49 |
| 5 | CF3 | CH3 | 0.43 | -0.069 | 0.36 |
| 6 | CF3 | C(CH3)3 | 0.43 | -0.1 | 0.33 |
| 7 | Ph | Ph | 0.06 | 0.06 | 0.12 |
| 8 | CH3 | Ph | -0.069 | 0.06 | -0.01 |
| 9 | CH3 | CH3 | -0.069 | -0.069 | -0.14 |
| 10 | C(CH3)3 | C(CH3)3 | -0.10 | -0.10 | -0.20 |
| 11 | Et | Et | -0.07 | -0.07 | -0.14 |
| 12 | Pr | Pr | -0.06 | -0.06 | -0.12 |
| 13 | Bu | Bu | -0.08 | -0.08 | -0.16 |
| 14 | iPr | iPr | -0.04 | -0.04 | -0.08 |
Ru(III) compounds 1 – 14 (Fig. 1) were optimized by different density functional theory (DFT) methods in the solvent phase (CH3CN). Table 2 and Table 3 lists the DFT solvent phase (CH3CN) computed data, namely the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies (EHOMO and ELUMO) and Mulliken electronegativity (χcalc, a measure of the tendency of an atom or molecule to attract electrons [3]) of the series of tris(β-diketonato)ruthenium(III) compounds 1 – 14 (Fig. 1). Experimental electrochemical data (potential E vs Fc/Fc+) of compounds 1 – 14, obtained from literature [4,5], are also given in Table 2. Different EHOMO, ELUMO and χcalc values are obtained by the different DFT methods, though all methods show the same trend, namely [Ru(β-diketonato)3] compounds containing electron withdrawing substituents on the β-diketonato ligand (e.g. complexes 1 – 6 containing a CF3 group) have lower EHOMO and ELUMO, and higher χcalc values than [Ru(β-diketonato)3] compounds containing electron donating substituents on the β-diketonato ligand (e.g. complexes 9 – 14), see Table 2 and Table 3.
Table 2.
DFT calculated data from this data article, as well as experimental electrochemical data (E vs Fc/Fc+) obtained from literature [4,5], of the [Ru(β-diketonato)3] compounds 1 – 14. Where β-diketonato ligand = (RCOCHCORꞌ)— with the R and Rꞌ substituents as shown in Fig. 1. DFT data was computed using two different gga functionals PW91 and OLYP.
| R | R' | E (RuIII/II)a | E (RuIII/IV)a | PW91/STO-TZ2P |
OLYP/STO-TZ2P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| EHOMO (eV) | ELUMO (eV) | χcalc (eV)c | EHOMO (eV) | ELUMO (eV) | χcalc (eV)b | |||||
| 1 | CF3 | CF3 | 0.34 | -5.856 | -5.585 | 5.720 | -5.573 | -5.337 | 5.455 | |
| 2 | CF3 | C4H3O | -0.34 | 1.20 | -5.067 | -4.744 | 4.906 | -4.793 | -4.522 | 4.657 |
| 3 | CF3 | C4H3S | -0.35 | 1.19 | -5.014 | -4.753 | 4.883 | -4.766 | -4.486 | 4.626 |
| 4 | CF3 | Ph | -0.35 | 1.26 | -5.134 | -4.838 | 4.986 | -4.865 | -4.606 | 4.736 |
| 5 | CF3 | CH3 | -0.47 | 1.29 | -5.132 | -4.812 | 4.972 | -4.869 | -4.596 | 4.733 |
| 6 | CF3 | C(CH3)3 | -0.55 | 1.30 | -5.065 | -4.777 | 4.921 | -4.782 | -4.513 | 4.648 |
| 7 | Ph | Ph | -0.90 | 0.66 | -4.622 | -4.294 | 4.458 | -4.339 | -4.070 | 4.205 |
| 8 | CH3 | Ph | -1.04 | 0.64 | -4.529 | -4.193 | 4.361 | -4.260 | -3.969 | 4.114 |
| 9 | CH3 | CH3 | -1.16 | 0.61 | -4.437 | -4.137 | 4.287 | -4.176 | -3.911 | 4.044 |
| 10 | C(CH3)3 | C(CH3)3 | -1.46 | 0.44 | -4.125 | -3.843 | 3.984 | -3.823 | -3.581 | 3.702 |
| 11 | Et | Et | -1.308 | 0.549 | -4.335 | -4.004 | 4.170 | -4.056 | -3.785 | 3.921 |
| 12 | Pr | Pr | -1.324 | 0.547 | -4.316 | -4.001 | 4.158 | -4.039 | -3.768 | 3.903 |
| 13 | Bu | Bu | -1.330 | 0.535 | -4.307 | -3.979 | 4.143 | -4.024 | -3.766 | 3.895 |
| 14 | iPr | iPr | -1.392 | 0.509 | -4.196 | -3.930 | 4.063 | -3.900 | -3.682 | 3.791 |
Experimental values for E vs Fc/Fc+ from references [4,5]. In order to convert to potential vs Fc/Fc+ for comparative reasons, the following values have been used:
E°' (Fc/Fc+) = 0.66(5) V vs NHE in solvent [n(Bu4)N][PF6]/CH3CN [9]; Saturated calomel (SCE) = 0.2444 V vs NHE; Ag/Ag+ = 0.400 V vs SCE [10].
χ = Electronegativity
Table 3.
DFT calculated data from this data article, as well as experimental electrochemical data (E vs Fc/Fc+) obtained from literature [4,5], of the [Ru(β-diketonato)3] compounds 1 – 14. Where β-diketonato ligand = (RCOCHCORꞌ)— with the R and Rꞌ substituents as shown in Fig. 1. DFT data was computed using two different hybrid functionals OPBE0 and B3LYP.
| R | R' | E (RuIII/II) a | E (RuIII/IV) a | OPBE0/STO-TZ2P |
B3LYP/STO-TZ2P |
B3LYP/STO-6-311G(d,p)/Lanl2dz |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHOMO (eV) | ELUMO (eV) | χcalc (eV) c | EHOMO (eV) | ELUMO (eV) | χcalc (eV) c | EHOMO (eV) | ELUMO (eV) | χcalc (eV) c | |||||
| 1 | CF3 | CF3 | 0.34 | -7.183 | -4.200 | 5.692 | -6.938 | -4.558 | 5.748 | -7.507 | -3.272 | 5.389 | |
| 2 | CF3 | C4H3O | -0.34 | 1.20 | -6.404 | -3.408 | 4.906 | -6.103 | -3.725 | 4.914 | -6.607 | -2.768 | 4.688 |
| 3 | CF3 | C4H3S | -0.35 | 1.19 | -6.393 | -3.410 | 4.901 | -6.074 | -3.707 | 4.890 | -6.635 | -2.822 | 4.729 |
| 4 | CF3 | Ph | -0.35 | 1.26 | -6.528 | -3.482 | 5.005 | -6.168 | -3.765 | 4.967 | -6.777 | -2.758 | 4.767 |
| 5 | CF3 | CH3 | -0.47 | 1.29 | -6.519 | -3.413 | 4.966 | -6.211 | -3.792 | 5.001 | -6.815 | -2.414 | 4.615 |
| 6 | CF3 | C(CH3)3 | -0.55 | 1.30 | -6.420 | -3.341 | 4.881 | -6.165 | -3.732 | 4.949 | -6.745 | -2.353 | 4.549 |
| 7 | Ph | Ph | -0.90 | 0.66 | -5.987 | -2.978 | 4.483 | -5.620 | -3.211 | 4.415 | -6.229 | -2.405 | 4.317 |
| 8 | CH3 | Ph | -1.04 | 0.64 | -5.922 | -2.863 | 4.393 | -5.595 | -3.132 | 4.364 | -6.216 | -2.137 | 4.177 |
| 9 | CH3 | CH3 | -1.16 | 0.61 | -5.863 | -2.719 | 4.291 | -5.215 | -2.785 | 4.000 | -6.166 | -1.634 | 3.900 |
| 10 | C(CH3)3 | C(CH3)3 | -1.46 | 0.44 | -5.467 | -2.403 | 3.935 | -5.215 | -2.785 | 4.000 | -6.021 | -1.474 | 3.748 |
| 11 | Et | Et | -1.308 | 0.549 | -5.722 | -2.588 | 4.155 | -5.435 | -2.962 | 4.199 | -6.151 | -1.648 | 3.900 |
| 12 | Pr | Pr | -1.324 | 0.547 | -5.703 | -2.577 | 4.140 | -5.415 | -2.938 | 4.177 | -6.136 | -1.633 | 3.884 |
| 13 | Bu | Bu | -1.330 | 0.535 | -5.681 | -2.565 | 4.123 | -5.415 | -2.946 | 4.180 | -6.130 | -1.619 | 3.874 |
| 14 | iPr | iPr | -1.392 | 0.509 | -5.523 | -2.470 | 3.996 | -5.289 | -2.836 | 4.062 | -6.123 | -1.591 | 3.857 |
Experimental values for E vs Fc/Fc+ from references [4,5]. In order to convert to potential vs Fc/Fc+ for comparative reasons, the following values have been used:
E°' (Fc/Fc+) = 0.66(5) V vs NHE in solvent [n(Bu4)N][PF6]/CH3CN [9]; Saturated calomel (SCE) = 0.2444 V vs NHE; Ag/Ag+ = 0.400 V vs SCE [10].
bχ = Electronegativity
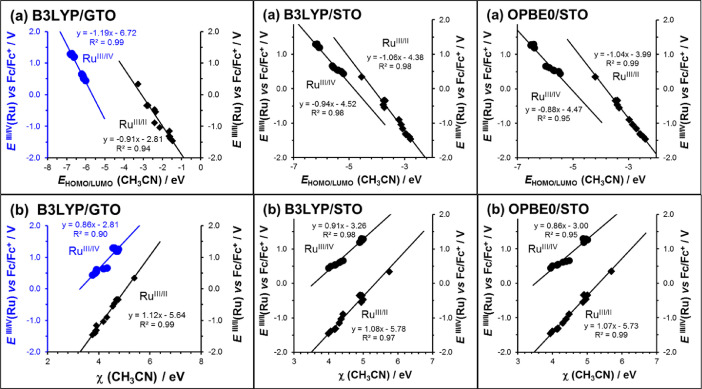
The relationships between the experimental values of the reduction (RuIII/II) and oxidation (RuIII/IV) couples [4,5] and the solvent (CH3CN) phase calculated EHOMO and ELUMO energies and their χcalc and ωcalc values, obtained via different DFT methods using generalized gradient approximations (gga) functionals, PW91/TZ2P and OLYP/TZ2P, are shown in Fig. 2 and obtained via different DFT methods using hybrid functionals, B3LYP/6-311G(d,p)/Lanl2dz, B3LYP/TZ2P and OPBE0/TZ2P, are shown in Fig. 3. The relationships obtained by these different solvent phase DFT methods, taking the experimental solvent (CH3CN) used for electrochemical experiments [4,5] into account in the calculations, all produced similar R2 values, comparable with the gas phase B3LYP/6-311G(d,p)/Lanl2dz calculated relationships obtained from reference [1]. The slopes of the experimental RuIII/II and RuIII/IV redox values versus the solvent (CH3CN) phase calculated EHOMO and ELUMO energies, are steeper than the corresponding gas phase calculated slope and also closer to nearing a gradient of -1.
Fig. 2.
Relationships obtained between the experimental redox potential E°' (vs Fc/Fc+) of both the reduction (RuIII/II) and the oxidation (RuIII/IV) redox couples of the fourteen [Ru(β-diketonato)3] compounds 1 – 14 of this data article, with the DFT calculated data, namely (a) the LUMO (RuIII/II) and HOMO (RuIII/IV) energies EHOMO/LUMO, (b) calculated Mulliken electronegativity χ. All calculations were conducted in CH3CN as solvent, using the indicated gga functionals.
Fig. 3.
Relationships obtained between the experimental redox potential E°' (vs Fc/Fc+) of both the reduction (RuIII/II) and the oxidation (RuIII/IV) redox couples of the fourteen [Ru(β-diketonato)3] compounds 1 – 14 of this data article, with the DFT calculated data, namely (a) the LUMO (RuIII/II) and HOMO (RuIII/IV) energies EHOMO/LUMO and (b) calculated Mulliken electronegativity χ. All calculations were conducted in CH3CN as solvent, using the indicated hybrid functionals.
Redox potentials and frontier orbital energies
Oxidation redox couple RuIII/IV:
| E°'(RuIII/IV) = -0.80 EHOMO(RuIII) – 4.00 | R2 = 0.98 | (gas phase B3LYP/6-311G(d,p)/Lanl2dz) [1] |
| E°'(RuIII/IV) = -1.19 EHOMO(RuIII) – 6.72 | R2 = 0.99 | (CH3CN phase B3LYP/6-311G(d,p)/Lanl2dz) |
| E°'(RuIII/IV) = -0.94 EHOMO(RuIII) – 4.52 | R2 = 0.98 | (CH3CN phase B3LYP/TZ2P) |
| E°'(RuIII/IV) = -0.88 EHOMO(RuIII) – 4.47 | R2 = 0.95 | (CH3CN phase OPBE0/TZ2P) |
| E°'(RuIII/IV) = -0.90 EHOMO(RuIII) – 3.33 | R2 = 0.96 | (CH3CN phase PW91/TZ2P) |
| E°'(RuIII/IV) = -0.87 EHOMO(RuIII) – 2.98 | R2 = 0.96 | (CH3CN phase OLYP/TZ2P) |
Reduction redox couple RuIII/II:
| E°'(RuIII/II) = -0.72 ELUMO(RuIII) – 2.21 | R2 = 0.98 | (gas phase B3LYP/6-311G(d,p)/Lanl2dz) [1] |
| E°'(RuIII/II) = -0.91 ELUMO(RuIII) – 2.81 | R2 = 0.94 | (CH3CN phase B3LYP/6-311G(d,p)/Lanl2dz) |
| E°'(RuIII/II) = -1.06 ELUMO(RuIII) – 4.38 | R2 = 0.98 | (CH3CN phase B3LYP/TZ2P) |
| E°'(RuIII/II) = -1.04 ELUMO(RuIII) – 3.99 | R2 = 0.99 | (CH3CN phase OPBE0/TZ2P) |
| E°'(RuIII/II) = -1.08 ELUMO(RuIII) – 5.59 | R2 = 0.98 | (CH3CN phase PW91/TZ2P) |
| E°'(RuIII/II) = -1.08 ELUMO(RuIII) – 5.36 | R2 = 0.98 | (CH3CN phase OLYP/TZ2P) |
HOMO (LUMO) energies are directly related to the absolute oxidation potential since the product of the HOMO (LUMO) energies (in eV) and the electron charge (-1) gives absolute oxidation potential in eV [6]. The nearer the slope of the graph of oxidation (reduction) potential versus HOMO (LUMO) energies is to -1, the more accurate the DFT method used to calculate the HOMO (LUMO) energies. The intercept of the graph should be equal to the absolute potential of reference used, namely the Fc+/Fc couple in acetonitrile for which benchmark values varies between +4.97 V (SMDB3LYP-D2/def2-QZVPPD//B3LYP/LanL2TZf/6-31G(d)) [7] and 4.988 V (G3(MP2)-RAD-Full-TZ using gas-phase energies and COSMO-RS solvation energies) [8]. In this study slopes of 0.7 – 1.2 and intercepts of 2.21 – 6.71 are obtained.
Redox potentials and global Mulliken electronegativity:
Oxidation redox couple RuIII/IV:
| E°'(RuIII/IV) = 0.67 χcalc – 1.80 | R2 = 0.96 | (gas phase B3LYP/6-311G(d,p)/Lanl2dz) [1] |
| E°'(RuIII/IV) = 0.86 χcalc – 2.81 | R2 = 0.90 | (CH3CN phase B3LYP/6-311G(d,p)/Lanl2dz) |
| E°'(RuIII/IV) = 0.91 χcalc – 3.26 | R2 = 0.98 | (CH3CN phase B3LYP/TZ2P) |
| E°'(RuIII/IV) = 0.86 χcalc – 3.00 | R2 = 0.95 | (CH3CN phase OPBE0/TZ2P) |
| E°'(RuIII/IV) = 0.90 χcalc – 3.19 | R2 = 0.97 | (CH3CN phase PW91/TZ2P) |
| E°'(RuIII/IV) = 0.89 χcalc – 2.91 | R2 = 0.96 | (CH3CN phase OLYP/TZ2P) |
Reduction redox couple RuIII/II:
| E°'(RuIII/II) = 0.81 χcalc – 4.09 | R2 = 0.98 | (gas phase B3LYP/6-311G(d,p)/Lanl2dz) [1] |
| E°'(RuIII/II) = 1.12 χcalc – 5.64 | R2 = 0.99 | (CH3CN phase B3LYP/6-311G(d,p)/Lanl2dz) |
| E°'(RuIII/II) = 1.08 χcalc – 5.78 | R2 = 0.97 | (CH3CN phase B3LYP/TZ2P) |
| E°'(RuIII/II) = 1.07 χcalc – 5.93 | R2 = 0.99 | (CH3CN phase OPBE0/TZ2P) |
| E°'(RuIII/II) = 1.09 χcalc – 5.79 | R2 = 0.98 | (CH3CN phase PW91/TZ2P) |
| E°'(RuIII/II) = 1.08 χcalc – 5.51 | R2 = 0.98 | (CH3CN phase OLYP/TZ2P) |
The energies relative to the ground state energy for the different possible spin states of the neutral, oxidized and reduced [Ru(acetylacetonato)3] compound 9 are provided in Table 4. The lowest energy value for each spin state showed that the neutral compound is low spin, S = ½ (doublet, one unpaired electron), in agreement with experiment [11]. The anion is diamagnetic with S = 0 (singlet), and the cation is paramagnetic with S = 1 (triplet, two unpaired electrons).
Table 4.
DFT calculated relative energy (eV) data obtained from this data article, for the different possible spin states of the neutral, oxidized and reduced [Ru(acetylacetonato)3], complex 9. The lowest energy value for each of the neutral, oxidized and reduced states, is taken as 0.
| Spin | B3LYP | PW91 | |
|---|---|---|---|
| anion | 0 | 0.00 | 0.00 |
| 1 | 1.27 | 1.51 | |
| 2 | - | 2.41 | |
| neutral | 1/2 | 0.00 | 0.00 |
| 3/2 | 1.42 | 1.61 | |
| 5/3 | 2.15 | 3.84 | |
| cation | 0 | 0.40 | 0.27 |
| 1 | 0.00 | 0.00 | |
| 2 | 0.00 | 1.79 |
2. Experimental Design, Materials, and Methods
DFT calculations on all fourteen [Ru(β-diketonato)3] compounds were performed in the CH3CN solvent phase, using the following DFT methods:
-
(i)
B3LYP/GTO-6-311G(d,p)/Lanl2dz: The hybrid functional B3LYP, which is composed of the Becke 88 exchange functional was applied in combination with the LYP correlation functional, as implemented in the Gaussian 16 package [12], applying the GTO (Gaussian type orbital) triple-ζ basis set 6-311G(d,p) for the lighter atoms (C, H, N, O, F) and the Lanl2dz (Los Alamos National Laboratory 2-double-ζ) basis set for the heavier Ru metal. The optimization is performed using Berny algorithm using GEDIIS [13] as implemented in the Gaussian 16 suite of programs [12]. The convergence is reached when the root mean square force, the maximum force, the root mean square displacement and the maximum displacement are within the threshold of 0.00030, 0.00045, 0.0012 and 0.0018 atomic units, respectively. The requested convergence on energy is 1.0D-6 atomic unit. The solvation model density (SMD) of the polarizable continuum model (PCM) was used, which also solved the non-homogeneous Poisson equation, by applying the integral equation formalism variant (IEF-PCM), as implemented in the Gaussian 16 package [12].
-
(ii)
PW91/STO-TZ2P: Scalar-relativistic DFT using the gga PW91 (Perdew-Wang 1991) functional with the all-electron STO (Slater-Type Orbitals) triple ζ basis set with two polarization functions (TZ2P) was applied, as implemented in the ADF 2018 package [14]. The geometry optimizations procedure in ADF is based on a quasi Newton approach, with an approximate Hessian. The Hessian is updated in the process of optimization. By default delocalized coordinates are used. The default convergence criteria were used, namely 10−3 Hartree for the energy and 10−3 Hartree/Angstrom for the nuclear gradients. Solvent effects were taken into account for selected structures reported here, using the COSMO (Conductor like Screening Model) model of solvation, as implemented [15] in ADF. The type of cavity used was Esurf and the solvent used was CH3CN (ε0 = 37.5).
-
(iii)
OLYP/STO-TZ2P: The gga OLYP functional was applied, with the TZ2P basis set and COSMO solvent model, as implemented in the ADF 2018 package [14].
-
(iv)
OPBE0/STO-TZ2P: The hybrid OPBE0 functional was applied, with the TZ2P basis set and COSMO solvent model, as implemented in the ADF 2018 package [14].
-
(v)
B3LYP/STO-TZ2P: The hybrid B3LYP functional was applied, with the TZ2P basis set and COSMO solvent model, as implemented in the ADF 2018 package [14].
The [Ru(β-diketonato)3] compounds were calculated as doublets (with S = ½) [11]. The input coordinates for the compounds were constructed using the program ChemCraft [16], and ChemCraft was also used to visualize the output files. The optimized coordinates, as well as an example input file, are provided in the supplementary information.
The DFT highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies (EHOMO and ELUMO) were obtained from the output file of the DFT computations. These energies were used to further calculate both the electron affinity (EA) and ionization potential (IP) of each of the fourteen compounds, according to Koopman's theorem [17,18]:
and
The Mulliken electronegativity (χ) [19] [20] was computed for each compound, by application of the following formulae:
For the unsymmetrically substituted compounds 2 – 6 and 8 where R ≠ Rꞌ, an effective calculated energy (EHOMO and ELUMO) was determined by using the ratio of the relative population of the fac and mer isomers (ni or nj), as determined by the Boltzmann equation at T = 298.15 K:
where ni is the number of molecules with energy Ei (fac or mer in this case), with the Boltzmann's constant, k = 1.38066 × 1023 JK−1. Ei are provided in the supplementary information.
Acknowledgments
This work has received support from the South African National Research Foundation (Grant numbers 113327 and 96111) and the Central Research Fund of the University of the Free State, Bloemfontein, South Africa. The CHPC of South Africa and the High Performance Computing facility of the UFS are acknowledged for computer time.
Conflict of Interest
The author declares that there is no conflict of interest regarding the publication of this article.
Footnotes
Supplementary material associated with this article, electronic energies, example input and output files, as well as the optimized coordinates of DFT calculations. can be found in the online version, at doi:10.1016/j.dib.2020.105617.
Appendix. Supplementary materials
References
- 1.Conradie J. Redox behaviour of [Ru(β-diketonato)3] compounds. Electrochim. Acta. 2020;337 doi: 10.1016/j.electacta.2020.135801. [DOI] [Google Scholar]
- 2.Hansch C., Leo A., Taft R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991;91:165–195. doi: 10.1021/cr00002a004. [DOI] [Google Scholar]
- 3.Mulliken R.S. A new electroaffinity scale; Together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 1934;2:782–793. doi: 10.1063/1.1749394. [DOI] [Google Scholar]
- 4.Takeuchi Y., Endo A., Shimizu K., Satô G.P. Electrochemical oxidation of tris(β-diketonato)-ruthenium(III) in acetonitrile solutions at platinum electrodes. J. Electroanal. Chem. 1985;185:185–189. doi: 10.1016/0368-1874(85)85852-4. [DOI] [Google Scholar]
- 5.Sharpe P., Alameddin N.G., Richardson D.E. Alkyl Substituent Effects in the Redox Thermochemistry of Coordination Compounds: Oxidation and Reduction Energetics for Ruthenium Tris(β-diketonate) Complexes in Solution and the Gas Phase. J. Am. Chem. Soc. 1994;116:11098–11108. doi: 10.1021/ja00103a027. [DOI] [Google Scholar]
- 6.Perdew J.P., Levy M. Comment on “Significance of the highest occupied Kohn-Sham eigenvalue,”. Phys. Rev. B. 1997;56:16021–16028. doi: 10.1103/PhysRevB.56.16021. [DOI] [Google Scholar]
- 7.Romańczyk P.P., Rotko G., Kurek S.S. The redox potential of the phenyl radical/anion couple and the effect thereon of the lithium cation: A computational study. Electrochem. Commun. 2014;48:21–23. doi: 10.1016/j.elecom.2014.08.003. [DOI] [Google Scholar]
- 8.Namazian M., Lin C.Y., Coote M.L. Benchmark Calculations of Absolute Reduction Potential of Ferricinium / Ferrocene Couple in Nonaqueous Solutions. J. Chem. Theory Comput. 2010;6:2721–2725. doi: 10.1021/ct1003252. [DOI] [PubMed] [Google Scholar]
- 9.Pombeiro A.J.L. Electron-donor/acceptor properties of carbynes, carbenes, vinylidenes, allenylidenes and alkynyls as measured by electrochemical ligand parameters. J. Organomet. Chem. 2005;690:6021–6040. doi: 10.1016/j.jorganchem.2005.07.111. [DOI] [Google Scholar]
- 10.S. Patai, The Chemistry of Metal Enolates, 2006. www.interscience.wiley.com.
- 11.Grobelny R., Jeowska-Trzebiatowska B., Wojciechowski W. The absorption spectra and magnetic properties of the chelated compounds of Ru(III) with β-diketones. J. Inorg. Nucl. Chem. 1966;28:2715–2718. doi: 10.1016/0022-1902(66)80398-6. [DOI] [Google Scholar]
- 12.M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, G.A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B.G. Janesko, R. Gomperts, B. Mennucci, H.P. Hratchian, J. V. Ortiz, A.F. Izmaylov, J.L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V.G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. Montgomery, J. A., J.E. Peralta, F. Ogliaro, M.J. Bearpark, J.J. Heyd, E.N. Brothers, K.N. Kudin, V.N. Staroverov, T.A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A.P. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, J.M. Millam, M. Klene, C. Adamo, R. Cammi, J.W. Ochterski, R.L. Martin, K. Morokuma, O. Farkas, J.B. Foresman, D.J. Fox, Gaussian 16, Revision B.01, (2016) 2016.
- 13.Li X., Frisch M.J. Energy-Represented Direct Inversion in the Iterative Subspace within a Hybrid Geometry Optimization Method. J. Chem. Theory Comput. 2006;2:835–839. doi: 10.1021/ct050275a. [DOI] [PubMed] [Google Scholar]
- 14.te Velde G., Bickelhaupt F.M., Baerends E.J., Fonseca Guerra C., van Gisbergen S.J.A., Snijders J.G., Ziegler T. Chemistry with ADF. J. Comput. Chem. 2001;22:931–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
- 15.Pye C.C., Ziegler T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Accounts Theory, Comput. Model. (Theoretica Chim. Acta) 1999;101:396–408. doi: 10.1007/s002140050457. [DOI] [Google Scholar]
- 16.http://www.chemcraftprog.com/,http://www.chemcraftprog.com/.
- 17.Parr R.G., Szentpály L.V., Liu S., Hamel S., Duffy P., Casida M.E., Salahub D.R., Koopmans T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica. 2002;1:104–113. doi: 10.1016/s0031-8914(34)90011-2. [DOI] [Google Scholar]
- 18.Conradie J. A Frontier orbital energy approach to redox potentials. J. Phys. Conf. Ser. 2015;633 doi: 10.1088/1742-6596/633/1/012045. [DOI] [Google Scholar]
- 19.Putz M.V., Russo N., Sicilia E. About the Mulliken electronegativity in DFT. Theor. Chem. Acc. 2005;114:38–45. doi: 10.1007/s00214-005-0641-4. [DOI] [Google Scholar]
- 20.Parr R.G., Donnelly R.A., Levy M., Palke W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978;68:3801–3807. doi: 10.1063/1.436185. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.