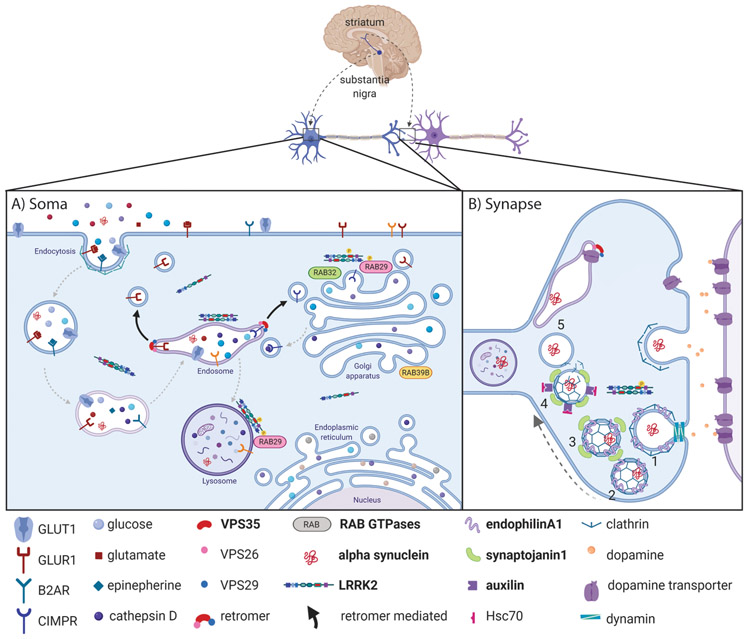
Figure 1:
PD-related proteins (bolded) in endolysosomal sorting pathways. A) In the neuronal soma ligands, receptors and transporters are taken up via endocytosis at the plasma membrane. As endosomes mature, lysosomal hydrolases, including cathepsin D, are delivered to the late endosome and lysosome from the trans-Golgi (TGN) as vesicles become more acidic (indicated by deepening purple). The retromer associates with early endosomes to identify cargo for retrograde transport (bold arrows) to the TGN or plasma membrane. Cargo that fail to be retrieved from endosomes accumulate and eventually undergo degradation in lysosomes. LRRK2 is cytoplasmic but is enriched upon different vesicular membranes in a dimeric, active conformation. Certain Rab GTPases are phosphorylated by LRRK2 with Rab29 regulating LRRK2 recruitment to the TGN and stressed lysosomes. PD-linked Rab32 and Rab39b may play a role within the Golgi complex. B) At the synapse, PD-associated proteins are responsible for clathrin-mediated synaptic vesicle endocytosis. 1) EndophilinA1 regulates membrane curvature and recruits dynamin-1 for constriction of plasma membrane buds and 2) eventual fission of clathrin-coated vesicles (CCV). 3) Synaptojanin-1 is recruited by endophilinA1 to dephosphorylate membrane lipids and release adaptor proteins (not shown), allowing auxilin to bind. 4) Auxilin and its cofactor Hsc70 remove the clathrin coat to produce synaptic vesicles that can fuse with endosomes or be loaded with neurotransmitters. Endocytosis represents one potential route for intake of misfolded or aggregated α-synuclein. Created using BioRender.com.