Figure 5.
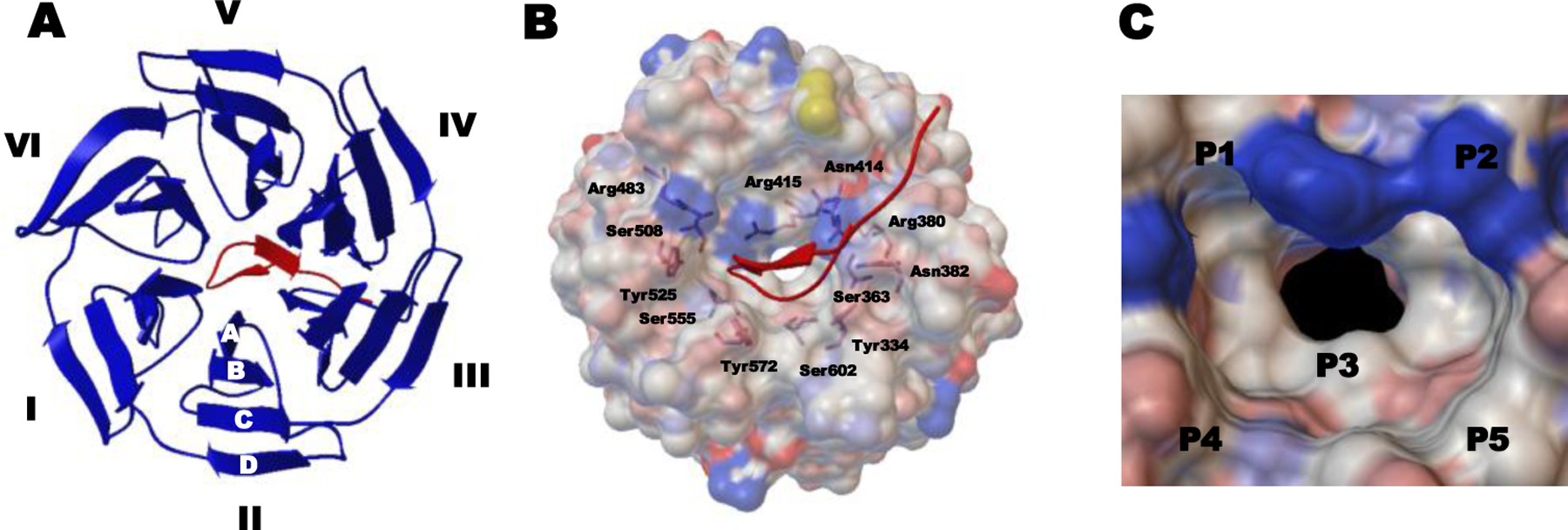
Co-crystal structure of the human Keap1 Kelch domain and the 16mer Nrf2-drived peptide containing the ETGE motif (PDB ID 2FLU). (A) Top view of the binding interaction of the Kelch domain with six blades I-VI and four β-strands A–D (shown as blue ribbon) and the Nrf2 peptide (shown as a red tube). (B) Full view of the interaction between the Keap1 Kelch domain (shown as a gray ele ctrostatic surface) and the Nrf2 peptide (shown as a red tube). Indicated residues are involved in interacting with the 16mer peptide. (C) Structure of the Keap1 binding cavity consisting of five subpockets (hotspots) P1–P5.
