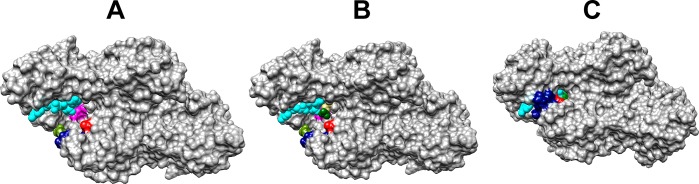
Figure 6.
Representations of the protein surface for binary and ternary complexes of hlGPDH: (A) binary complex with NAD (PDB entry 6E8Z), (B) binary complex with NAD with substrate DHAP inserted at the position observed for the ternary hlGPDH·NAD·DHAP complex, and (C) ternary hlGPDH·NAD·DHAP complex (PDB entry 6E90). Color key: NAD, cyan; flexible protein loop (292-LNGQKL-297), navy blue; Q295, olive; R269, red; K120, magenta; DHAP, dark green for the phosphodianion and khaki for the remainder of the substrate. Substrate binding is accompanied by a movement of the navy blue flexible protein loop that covers the substrate and cofactor in the closed enzyme, formation of a hydrogen bond between the Q295 (olive) and R269 (red) side chains, and movement of the R269 side chain toward the substrate phosphodianion (green) and the cofactor pyrophosphate (cyan). The buried K120 side chain and substrate phosphodianion are hidden from view in panel C.