Abstract
Background and Aims
There are currently several recruitment challenges in randomized controlled trials (RCTs) for inflammatory bowel disease (IBD), which prolong the drug approval process and affect the generalizability of study results. The purpose of this study is to characterize individuals who participate in IBD RCTs and identify factors that could influence future recruitment strategies.
Methods
We performed a cross-sectional study within the IBD Partners cohort comparing patients with current or prior participation in an RCT of medical therapy for IBD to those without any RCT participation. Bivariate statistics were used to compare RCT participation by IBD subtype and by other demographic and disease characteristics, and predictive modeling was used to identify factors predictive of RCT participation. We calculated the percent of the cohort that participated in an RCT during each calendar year from 2011 to 2018 and accessed Clinicaltrials.gov to determine the number of active RCTs for IBD therapies per year during that same period.
Results
A total of 14,747 patients with IBD were included in the analysis and 1116 (7.6%) reported RCT participation at any time. Demographic factors predictive of RCT participation included following at an academic institution [odds ratio (OR) = 1.8; 95% confidence interval (CI) 1.51–2.04) and age 36–75 (OR = 1.7; 95% CI 1.46–1.92). Patients with Crohn’s disease were more likely to participate than those with ulcerative colitis (OR = 1.5; 95% CI 1.35–1.77). Patients with more severe disease were more likely to participate, including those with prior IBD-related hospitalization (OR = 2.6; 95% CI 2.19–2.99), IBD-related surgery (OR = 2.5; 95% CI 2.24–2.87), biologic exposure (OR = 3.2; 95% CI 2.76–3.65), and “Poor” or worse quality of life (OR = 1.7; 95% CI 1.45–1.93). Steroid-free remission was associated with a lower likelihood of RCT participation (OR = 0.6; 95% CI 0.53–0.70). Although the number of active RCTs for IBD more than doubled between 2011 and 2018, RCT participation rates during that same time period decreased from 1.1% to 0.7% of the cohort.
Conclusions
RCT participation declined within this cohort. Groups underrepresented in RCTs for IBD included younger patients, patients followed in community settings, and patients with more mild disease. The non-RCT group had mean disease activity scores that did not meet remission thresholds, demonstrating populations in need of alternate therapies for whom clinical trials could be an option. Given anti-tumor necrosis factor (TNF) exposure rates in this national cohort, studies should focus on anti-TNF failure populations. Investigators should make every effort to offer RCTs to all patients and network with community providers to increase awareness of RCTs.
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis, clinical trials, randomized controlled trials, eligibility, generalizability, trends, characteristics, enrollment, participation
BACKGROUND
Since the Food and Drug Administration (FDA) approval of infliximab for Crohn’s disease (CD) in 1998, biologics have revolutionized the management of inflammatory bowel disease (IBD). Compelling evidence from numerous randomized controlled trials (RCTs) supports their safety and efficacy at inducing and maintaining remission,1,2 and other studies have shown improved outcomes such as surgeries, hospitalizations, and quality of life.3,4 In addition to monoclonal antibodies, other categories of new therapies have shown promise in inducing and maintaining remission of IBD. Despite the success of these therapies, a large proportion of IBD patients continue to have refractory disease. This therapy gap underscores the need for continued research and innovation.
Clinical trial recruitment and retention are often rate-limiting steps in the development of new therapies, and there are currently several challenges in IBD clinical trials recruitment. A recent review by Harris et al5 proposed several factors contributing to this problem, including an increased number of IBD drugs in development, a greater number of approved therapies available to patients, increasingly complex study endpoints and protocols, and a growing list of excluded concomitant medications. Meanwhile, pharmaceutical development for IBD continues at an impressive pace. In 2018, there were 11 new biologics in phase 2 or 3 clinical trials,6 and many more biosimilars and small molecule agents. These biologics collectively target 9 different molecules, 7 of which are novel targets for IBD. Unfortunately, most US sites enroll only 1–2 patients per IBD trial,5,7 prolonging the drug approval process while simultaneously raising questions about the generalizability of study results.
Little is known about overall participation in clinical trials for IBD in the United States. A better understanding of these characteristics may inform site selection, clinical trial inclusion criteria, and development of new protocols in the future. The purpose of this study is to quantify, characterize, and contextualize IBD clinical trials participation in a large registry of IBD patients. We also aimed to identify factors predictive of RCT participation. Although prior studies have attempted to characterize patient eligibility in IBD RCTs8,9 or identify patient barriers to RCT participation,10,11 there is no recent literature that has described IBD RCT participation on a large scale across the United States, including specific disease characteristics and site of care data. We also aimed to compare annual participation rates for RCTs in our study to currently available therapeutic trials in IBD to better understand the changing landscape. In order to maintain a population of study participants that can match the pace of discovery of new therapeutic agents, data that may inform expansion of participation in clinical trials will be critical.
METHODS
Study Design
We included patients enrolled in IBD Partners patient cohort in a cross-sectional analysis. We compared patients who reported current or previous participation in an RCT of a therapeutic medication for IBD to those with no participation. Briefly, IBD Partners is a large Internet-based cohort that includes more than 16,000 patients with self-reported IBD.12 Cohort members are followed up every 6 months with surveys covering a wide array of subjects, including demographics, disease phenotype and activity, medications, research participation, and patient-reported outcomes. The data were collected entirely in a Web-based format, allowing for real-time implementation of range and consistency checks. The details of the data management system for IBD Partners have been previously reported.12
Study Population
Patients were included in the analysis if they had completed the clinical trial questionnaire in IBD Partners, reported CD or ulcerative colitis (UC) disease type, and resided in the United States.
Study Exposures
Patient demographics and disease-specific information including CD or UC disease type, disease duration, prior surgeries, hospitalizations, and medications were measured. Disease activity was assessed using short Crohn’s Disease Activity Index (sCDAI) for CD and Simple Clinical Colitis Activity Index (SCCAI) for UC.
For each participant, levels of anxiety, depression, fatigue, sleep disturbances, pain interference, and social satisfaction were assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS). The PROMIS measures have been previously validated in general in chronically ill populations and their performance was assessed within the IBD Partners cohort.13 In brief, participants completed 4 items for each of the PROMIS measures. The items were then calibrated using a T-score metric to provide a mean value for the measure assessed. Medications, medical history (eg, prior IBD-related surgery or hospitalization), and provider setting were also reported.
Study Outcome
Clinical trial participation was defined as ever or current participation in a clinical trial of a therapeutic agent for IBD.
Statistical Analysis
Descriptive statistics were used to characterize the population, including proportions and 95% confidence intervals (CIs), medians and interquartile ranges, means and standard deviations (SDs) as appropriate. Bivariate statistics were used to compare RCT participation and non-RCT population and stratified by IBD subtype (CD vs UC) and by other demographic and disease characteristics. These statistics included Pearson’s chi-square test statistic and Student’s t-test as appropriate. Logistic regression was used to examine the relationship between RCT participation (0/1) as the dependent variable and possible predictive factors as the independent variables. The coefficients obtained from the logistic regression analysis were expressed with odds ratios (ORs). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
To determine the number of RCTs available for IBD in a given year, we searched Clinicaltrials.gov for all past and current phase 2 and 3 industry-funded interventional studies for IBD in the United States. The results were exported into Microsoft Excel and were filtered by study start date and primary completion date (or termination/suspension date, if applicable) to determine the number of active trials on June 30 of each year between 2011 and 2018. Trials with missing or null start dates and primary completion dates were excluded.
To determine annual RCT participation prevalence from 2011 to 2018, current RCT-participation status was assessed serially in a cross-sectional fashion each year. Participation in an RCT was based on self-reported use of clinical trial medication in the “IBD Medications” survey in IBD Partners, which patients may update at any time. The annual prevalence was calculated by dividing the number of patients who reported currently using a clinical trial medication by the number of patients who completed an IBD Partners survey during each calendar year. This was a longitudinal analysis in that an individual patient could contribute to multiple years, but patients who reported multiple RCT interventions in a given year were counted only once for that year.
The study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
RESULTS
Cohort Characteristics
There were 16,441 patients enrolled in IBD Partners at the time of data collection and 14,747 met the inclusion criteria and were included in the analysis (Table 1). A total of 1116 (7.6%) patients reported having taken a clinical trial medication at any time and were included in the RCT-participant group. The remaining 13,628 (92.4%) were included in the comparison group.
TABLE 1.
Attrition
| Step | Description | Patients, n (%) |
|---|---|---|
| 1 | Total patients in the IBD Partners cohort (as of June 30, 2019) | 16,441 |
| 2 | Patients having completed the clinical trial questionnaire at any visit | 16,079 (97.8%) |
| 3 | Patients reporting either CD or UC diagnosis | 15,662 (97.4%) |
| 4 | Patients reporting residence in the United States | 14,747 (94.2%) |
| 4a | Patients ever having reported taken or currently taking a clinical trial medication | 1119 (7.6%) |
| 4b | Patients never having reported taken or currently taking a clinical trial medication | 13,628 (92.4%) |
Demographic and disease characteristics of the RCT-participant and nonparticipant groups are detailed in Table 2. The groups were equivalent based on sex (P = 0.575), race (P = 0.647), and Hispanic ethnicity (P = 0.143). The RCT-participant group was slightly older at 45.5 ± 14.1 years (P < 0.001). The RCT-participant group had a greater proportion of CD patients at 72.9% (P < 0.001). More participants were followed by gastroenterologists at academic centers than nonparticipants (P < 0.001). RCT participants reported more prior IBD-related hospitalizations (P < 0.001) and higher rates of use of every category of IBD medication, especially immunomodulators and biologics. Among RCT participants, 75.3% reported prior exposure to anti-tumor necrosis factor (TNF) agents (P < 0.001), 36.6% to vedolizumab (P < 0.001), 25.6% to ustekinumab (P < 0.001), and 14.0% to natalizumab (P < 0.001). RCT participants reported worse PROMIS depression (P = 0.007), fatigue (P < 0.001), pain interference (P > 0.001), sleep disturbance (P < 0.001), and social satisfaction scores (P < 0.001). A greater proportion of RCT participants reported feeling Poor, Very Poor, and Terrible on the General Well Being Index (P < 0.001). RCT participants had a higher mean sCDAI score (187.0 vs 153.6; P < 0.001) and SCCAI score (4.4 vs 3.8; P = 0.002) and a lower steroid-free remission rate (34.5% vs 46.4%; P < 0.001).
TABLE 2.
Demographics and Clinical Characteristics Among RCT Participants and Non-RCT Participants Enrolled in IBD Partners
| RCT Participants | Non-RCT Participants | P | |
|---|---|---|---|
| Total patients, n (%) | 1119 (7.6%) | 13,628 (92.4%) | |
| Female (%) | 71.1% | 71.9% | 0.575 |
| Disease classification (% CDa) | 72.9% | 63.5% | <0.001 |
| Ageb, mean (SD) | 45.5 (14.1) | 42.2 (14.8) | <0.001 |
| 18–35 | 27.7% | 39.2% | |
| 36–74 | 70.7% | 59.6% | |
| 75+ | 1.3% | 1.1% | |
| Race (%) | 0.647 | ||
| Black/African American | 1.9% | 2.3% | |
| White/Caucasian | 89.4% | 88.7% | |
| Other/missing | 8.8% | 9.0% | |
| Hispanic/Latino ethnicity (%) | 2.3% | 3.1% | 0.143 |
| College education (%)c | 89.7% | 88.6% | 0.037 |
| University/academic practice (%) | 22.7% | 14.5% | <0.001 |
| Medical history | |||
| Total hospitalizations, mean (SD) | 4.3 (2.1) | 3.3 (1.9) | <0.001 |
| IBD-related hospitalization (%) | 81.3% | 63.0% | <0.001 |
| IBD-related surgery (%) | 58.6% | 35.9% | <0.001 |
| IBD medication history | |||
| Steroid exposure (%) | 97.2% | 90.1% | <0.001 |
| 5-ASA exposure (%) | 96.1% | 89.4% | <0.001 |
| Anti-TNF exposure (%) | 75.3% | 49.5% | <0.001 |
| Vedolizumab exposure (%) | 36.6% | 7.6% | <0.001 |
| Ustekinumab exposure (%) | 25.6% | 5.8% | <0.001 |
| Natalizumab exposure (%) | 14.0% | 0.8% | <0.001 |
| “Poor” or worse in Well-Beingd (%) | 26.1% | 17.4% | <0.001 |
| PROMISeT-score, mean (SD) | |||
| Anxiety | 54.3 (9.7) | 54 (9.7) | 0.285 |
| Depression | 52.8 (9.6) | 51.9 (9.6) | 0.007 |
| Fatigue | 58.1 (10.5) | 55.8 (11.0) | <0.001 |
| Pain inteference | 55.6 (10.7) | 53.7 (10.3) | <0.001 |
| Social satisfaction | 46.3 (10.1) | 47.9 (9.9) | <0.001 |
| Disease activity | |||
| Disease duration (years), mean (SD) | 19.7 (12.5) | 13.2 (12.11) | <0.001 |
| sCDAI score, mean (SD)f | 187.0 (113.9) | 153.6 (103.2) | <0.001 |
| SCCAI score, mean (SD)g | 4.4 (3.2) | 3.8 (3.0) | 0.002 |
| Steroid-free remission (%)h | 34.5% | 46.4% | <0.001 |
aCD, Crohn’s disease.
bFor RCT participants, age at first participation in a clinical trial; for non-RCT participants, age at first completed survey in IBD Partners.
cDefined as “some college” or more.
dResponse to the General Well-Being questionnaire on IBD Partners.
ePROMIS, Patient-reported outcome measurement information system measured in T-scores, with mean 50 and SD 10 in the general population. Higher scores signal more of the domain measured.
fsCDAI (short Crohn’s Disease Activity Index) assesses abdominal pain, stool patterns, and overall well-being, with increasing values indicating worse disease. Remission is defined by less than 150 points on a scale from 0 to 450.
gSCCAI (Simple Clinical Colitis Activity Index) assesses bowel frequency, urgency, hematochezia, general health, and extracolonic manifestations, with increasing values indicating worse disease. Remission is defined by ≤2 points on a scale from 0 to 19.
hDefined as no use of steroids and either sCDAI <150 or SCCAI ≤2.
Results were further stratified for CD and UC and are available in Supplementary Tables 2a and 2b, respectively. There was a significant difference in the education level between the CD groups (P = 0.016) but not the UC group (P = 0.983).
Factors Predictive of RCT Participation
Predictive factors for RCT participation are detailed in Table 3. With respect to patient demographics, patients followed at academic institutions were almost twice as likely to participate in RCTs than those in private or community practice (OR = 1.8; 95% CI 1.51–2.04). Age was predictive as well, as those in the 36–75 age group were more likely to have participated when compared to patients aged 18–35 (OR = 1.7; 95% CI 1.46–1.92). Sex, race, ethnicity, and education level were not predictive of participation.
TABLE 3.
Multivariable Analysis Demonstrating Factors Associated With Participation in RCTs
| Characteristics | Adjusted Odds Ratio (95% CI) |
|---|---|
| Crohn’s diseasea | 1.54 (1.35–1.77) |
| Age 36–75 vs 18–35 | 1.68 (1.46–1.92) |
| Age 75+ vs 18–35 | 1.68 (0.96–2.93) |
| College educationb | 1.09 (0.95–1.25) |
| White race | 1.07 (0.88–1.31) |
| Hispanic ethnicity | 0.74 (0.49–1.11) |
| University/academic setting | 1.76 (1.51–2.04) |
| Prior IBD-related hospitalization | 2.56 (2.19–2.99) |
| Prior IBD-related surgery | 2.53 (2.24–2.99) |
| Biologic exposure | 3.17 (2.76–3.65) |
| “Poor” or worse in General Well-Beingc | 1.67 (1.45–1.93) |
| Steroid-free remission | 0.61 (0.53–0.70) |
aVersus ulcerative colitis.
bDefined as “some college” or more.
cResponse to the General Well-Being questionnaire on IBD Partners.
Several disease characteristics were predictive of RCT participation. CD patients were more likely than UC patients to have participated in an RCT (OR = 1.5; 95% CI 1.35–1.77). Patients reporting indicators of more severe disease were more likely to have participated, including those with prior IBD-related hospitalization (OR = 2.6; 95% CI 2.19–2.99), IBD-related surgery (OR = 2.5; 95% CI 2.24–2.87), and biologic exposure (OR = 3.2; 95% CI 2.76–3.65). Likewise, steroid-free remission was associated with a lower likelihood of RCT participation (OR = 0.6; 95% CI 0.53–0.70). Quality of life had a significant impact as well, as patients reporting “Poor” or worse condition on the general well-being index were more likely to report participation (OR = 1.7; 95% CI 1.45–1.93).
Results were further stratified for CD and UC and are available in Supplementary Tables 3a and 3b, respectively. There were no differences in predictive factors between the CD and UC cohorts.
Active RCTs vs Participation Over Time
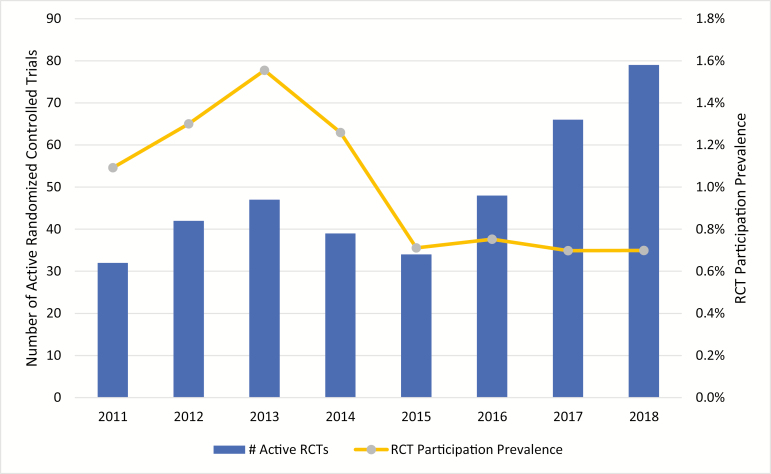
Our query on Clinicaltrials.gov returned 295 results for our search criteria. Eight were excluded for a null start or stop dates, yielding 287 RCTs. About 165 RCTs were active at some point between June 2011 and June 2018, with 81 (49.1%) of those being investigations for biologics or biosimilars. Our analysis of available RCTs for IBD over time revealed that there were 32 active RCTs in June 2011 and 79 in June 2018, reflecting an annualized growth rate of 13.8% (Figure 1). Meanwhile, rates of reported RCT participation in our cohort decreased during this same time period. In 2011, 1.1% of the IBD Partners reported current clinical trial participation compared to 0.7% in 2018 (Figure 1).
FIGURE 1.
Active IBD RCTs1 and IBD RCT participation prevalence2, 2011–2018. 1Active phases 2 and 3 industry-funded interventional studies for IBD in the United States as of June 30 of each year (source: clinicaltrials.gov). 2Calculated by dividing the number of patients who reported using a clinical trial medication by the number of patients who completed an IBD Partners survey during each calendar year.
DISCUSSION
In our study, we identified demographic trends in RCT participation that may present opportunities for enhanced and/or targeted marketing efforts. For example, we found that patients followed at academic centers were almost twice as likely to have participated in an RCT. However, we also found that over half of the RCT participants in our cohort were followed in private practice settings. This should be interpreted as an encouraging sign, because the proliferation of private-practice investigators makes RCTs more accessible to IBD patients, the vast majority of whom are not seen at major academic centers. In addition, academic investigators should make every effort to network with community providers to increase awareness of RCTs. We also found that patients aged 36–75 were more likely to have participated in RCTs, when compared to patients aged 18–35. Although not statistically significant patients older than age 75 also seemed more likely to participate in clinical trials than patients aged 18–35. Further recruitment directed at younger age groups could enhance participation. Finally, patients reporting RCT participation reported more advanced and/or complicated disease than the non-RCT group, with a worse quality of life. This is unsurprising, as one could argue that sicker patients might be more willing to try unproven therapies out of necessity or desperation (ie, they have failed all currently approved medical options). What is more surprising, however, is that the nonparticipant group also reported high rates of active disease and relatively poor quality of life, albeit not as extreme as the RCT group. Fewer than half of the non-RCT patients reported feeling “generally well” and the steroid-free remission rate was only 46%. In fact, neither the mean sCDAI nor the mean SCCAI for the non-RCT group met their respective remission thresholds. This underscores the fact that there are many RCT-naïve patients who are not responding to current standard-of-care therapies and reinforces the role for targeting patients with less severe disease.
Our data showed that the availability of IBD clinical trials in the United States has increased sharply over the past decade. From 2011 to 2018, the number of active RCTs for IBD therapies in a given year more than doubled. The trend was similar for biologic investigations and is consistent with results from another recent study that included RCTs conducted both in the United States and abroad.5 According to our analysis of the active RCTs, the rapid increase between 2011 and 2018 was driven primarily by the development of new biologics, biosimilars, and various small molecule drugs such as JAK–STAT pathway inhibitors. In fact, biologics and biosimilars accounted for nearly half (81/165; 49.1%) of all active RCTs during this time. Strikingly, the dramatic increase in active RCTs for IBD was not supported by increased RCT participation. In fact, RCT participation as a percentage of our study cohort actually declined during the same time period in the United States. Although the exact reason for this pattern was not evaluated qualitatively, the timing of FDA approvals of high-impact, first-in-class IBD therapies is likely responsible. For example, the precipitous drop in participation between 2013 and 2015 correlated with the FDA approval of vedolizumab in 2014.14 Despite a sharp increase in RCTs between 2015 and 2018, participation was stagnant, likely suppressed by the FDA approval of ustekinumab in 2016 and tofacitinib in 2018.14 With new treatment options on the table, it is logical to assume that many patients who had already failed anti-TNF therapy opted to try other approved agents before enrolling in a trial where they might receive placebo. Aside from blinding, previous studies have also identified frequent doctor visits, large time commitment, and colonoscopy requirements as barriers to enrollment.10,11
Our findings also reflect the need to rethink trends in RCT design for IBD in the coming years. In 2012, Ha et al8 found that only 31.1% of IBD patients at a tertiary care center would have qualified for any 1 of 8 major RCTs investigating new biologic therapies between 2002 and 2012. Several of these patients were rendered ineligible for having been exposed to anti-TNF agents in the past or for having signs of advanced disease. The number of patients receiving these therapies has only increased since then, with our results showing that more than 75% of RCT participants and 49.5% of nonparticipants in our cohort have prior anti-TNF exposure. Therefore, designing RCTs that exclude these patients is impractical because it fails to address a large area of need and will likely result in underpowered studies secondary to recruitment challenges. Based on our results, we believe comparative effectiveness studies should focus on the TNF-failure population, because this is likely the population for whom the investigational agents are most relevant. In order to compete with the ever-growing menu of approved therapy options, study design should incentivize patient participation whenever possible, such as by offering long-term open-label extensions that maximize potential benefit and minimize the risk of receiving placebo. A 2012 study on patient barriers to RCT enrollment found that 40% of those unwilling to participate in a trial indicated that they might be willing if the study had an open-label extension.10 This idea is further supported by other recent literature that has proposed favoring active comparator studies over placebo-controlled studies in RCTs for IBD.5,15
Another means of improving participation rates in clinical trials is to engage community centers who may see patients earlier in the disease course. These patients may meet eligibility criteria at higher rates than those seen at academic institutions. There are a number of available resources for enhancing such participation, including groups such as the Crohn’s and Colitis Foundation Clinical Research Alliance. This alliance includes academic and community sites interested in research and offers opportunities for observational and interventional research with shared infrastructure and support. Additionally, the foundation hosts a clinical trials finder, where patients can search for clinical trials in their geographic area. Resources for training community providers in clinical trials are needed, to enhance the numbers of participating community sites. Providing mentorship from academic investigators experienced in clinical trial operations could also potentially enhance community participation. Further efforts of expanding access to clinical trials in the community are needed in IBD.
The strengths of this study include the size of the cohort and the large degree of geographic diversity associated with it. Additionally, the sample included a majority of patients treated at community sites, which is the most common care model in the United States. The study also has existing limitations. The data included from IBD Partners are via self-report; however, a previous validation study of a sample of the cohort demonstrated that more than 97% had IBD based on medical record review.13 Our results may not be externally generalizable in that patients who voluntarily joined the IBD Partners cohort may be more likely to participate in other types of research such as RCTs. Notably, this would likely be a directional bias overestimating RCT participation, which emphasizes just how low RCT participation rates are, as participation never exceeded 1.6% in any given year we measured. Another limitation is that we did not capture patients’ impressions surrounding clinical trial participation and thus are unable to determine the impact of interventions such as placebo rates or monetary incentives. Finally, the overrepresentation of women and college-educated patients and underrepresentation of minorities in the IBD Partners cohort compared to population levels may impact the external validity of our results.
Recruitment for IBD RCTs has become increasingly difficult since 2014 and participation remains very low. Factors predictive of RCT participation include middle age, academic practice setting, more severe disease, and worse quality of life. Efforts should be made to recruit beyond these patient groups. Expanding the pool of potential participants will hopefully reduce the recruitment burden and produce results more generalizable to the real-world population of patients with IBD.
Supplementary Material
Supported by: Funding for this project was provided by a student research grant from National Institute of Diabetes and Digestive and Kidney Diseases (T35-DK007386).
Conflict of interest statement. E.L.B.: Consultant for AbbVie, Takeda, and TARGET Pharmasolutions. M.D.L.: Consultant for AbbVie, Takeda, UCB, Janssen, Pfizer, Salix, Prometheus, Valeant, and TARGET Pharmasolutions. Research support from Takeda and Pfizer.
REFERENCES
- 1. Chan HC, Ng SC. Emerging biologics in inflammatory bowel disease. J Gastroenterol. 2017;52:141–150. [DOI] [PubMed] [Google Scholar]
- 2. Ford AC, Khan KJ, Sandborn WJ, et al. . Once-daily dosing vs. conventional dosing schedule of mesalamine and relapse of quiescent ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:2070–2077; quiz 2078. [DOI] [PubMed] [Google Scholar]
- 3. Feagan BG, Panaccione R, Sandborn WJ, et al. . Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. [DOI] [PubMed] [Google Scholar]
- 4. Loftus EV, Feagan BG, Colombel JF, et al. . Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103:3132–3141. [DOI] [PubMed] [Google Scholar]
- 5. Harris, MS, Wichary J, Zadnik M, et al. . Competition for clinical trials in inflammatory bowel diseases. Gastroenterology. 2019;157:6:1457-1461. [DOI] [PubMed] [Google Scholar]
- 6. Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herfarth HH, Jackson S, Schliebe BG, et al. . Investigator-initiated IBD trials in the United States: facts, obstacles, and answers. Inflamm Bowel Dis. 2017;23:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ha C, Ullman TA, Siegel CA, et al. . Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–1007; quiz e78. [DOI] [PubMed] [Google Scholar]
- 9. Van Spall HG, Toren A, Kiss A, et al. . Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–1240. [DOI] [PubMed] [Google Scholar]
- 10. Ravikoff JE, Cole EB, Korzenik JR. Barriers to enrollment in inflammatory bowel disease randomized controlled trials: an investigation of patient perspectives. Inflamm Bowel Dis. 2012;18:2092–2098. [DOI] [PubMed] [Google Scholar]
- 11. Ehrlich OT, Testaverde J, Heller C, et al. . Crohn’s disease and ulcerative colitis patient perspectives on participation in clinical trials. Inflamm Bowel Dis. 2018;24(suppl 1):S9. [Google Scholar]
- 12. Long MD, Kappelman MD, Martin CF, et al. . Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis. 2012;18:2099–2106. [DOI] [PubMed] [Google Scholar]
- 13. Randell RL, Long MD, Cook SF, et al. . Validation of an internet-based cohort of inflammatory bowel disease (CCFA partners). Inflamm Bowel Dis. 2014;20:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crohn’s and Colitis Foundation. Recently Approved Treatments. New York, NY: Irwin M. Suzanne R. Rosenthal IBD Resource Center; 2019. [Google Scholar]
- 15. Danese S, Schabel E, Masure J, et al. . Are we ready to abandon placebo in randomised clinical trials for inflammatory bowel disease? Pros and cons. J Crohns Colitis. 2016;10(suppl 2):S548–S552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.