Table 2.
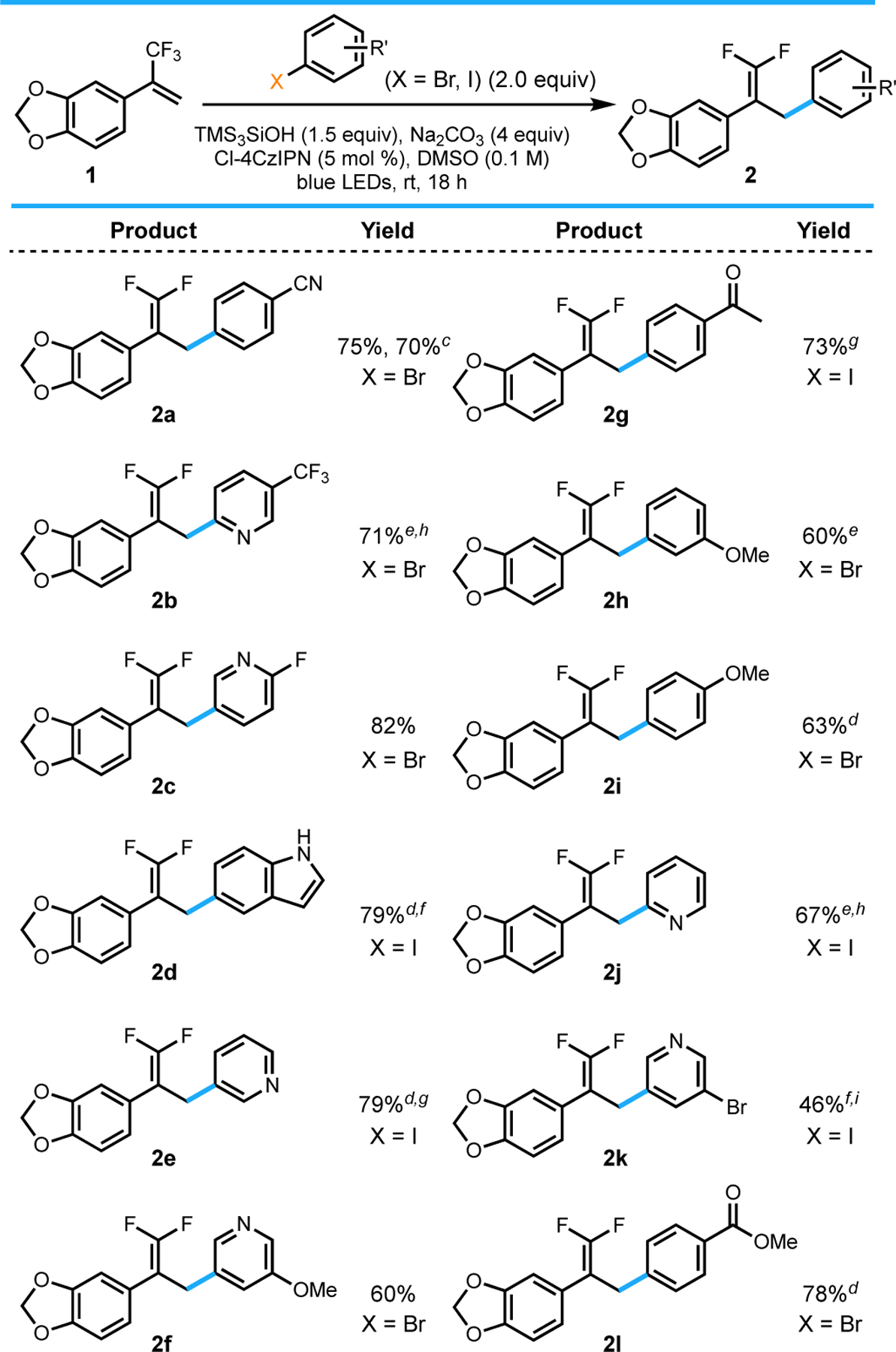 |
All values indicate the yield of the isolated product.
General reaction conditions: aryl halide (2.0 equiv, 1.0 mmol), alkene (1.0 equiv, 0.50 mmol), Cl-4CzIPN (5 mol %, 0.025 mmol), TMS3SiOH (1.5 equiv, 0.75 mmol), Na2CO3 (4 equiv, 2.0 mmol), DMSO (0.1 M), 18 h, irradiating with blue LEDs (30 W). See the Supporting Information for details.
Isolated yield on 2.5 mmol scale; reaction run for 5 d.
Isolated on 1.0 mmol scale.
Isolated yield on 0.3 mmol scale.
Conducted using 10 mol % Cl-4CzIPN.
Reaction run for 2 d.
Conducted using 1 equiv Ar–X. hIsolated with <5% impurity of alkene.
Isolated as a 7.6:1 mixture of Br:I arene.
