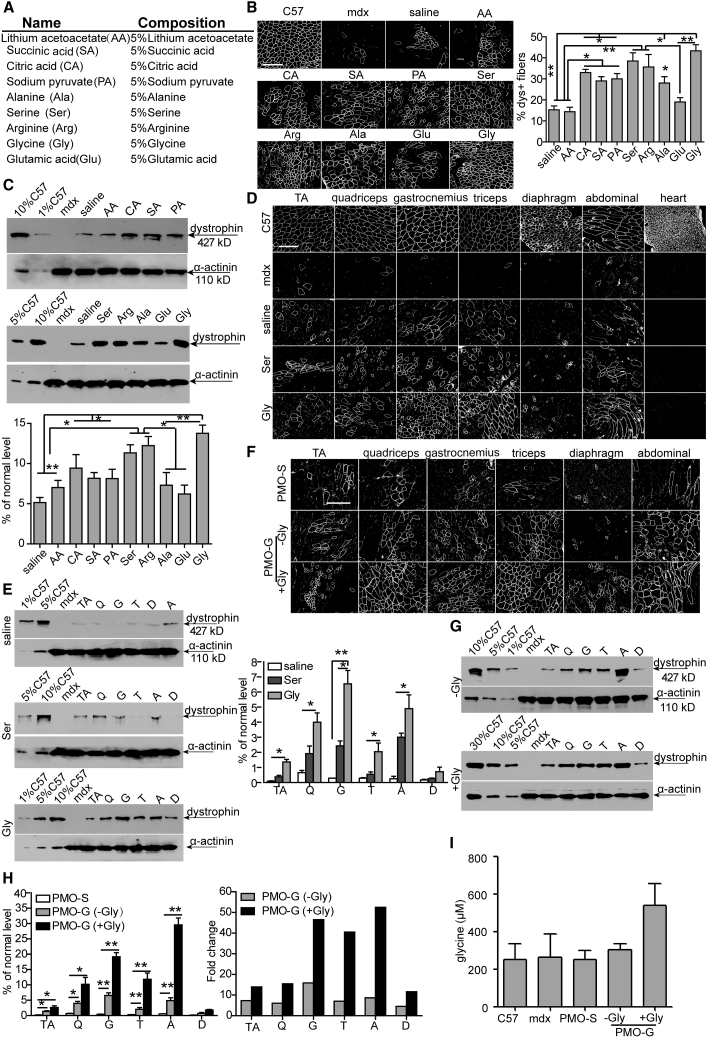
Figure 1.
Screening of Amino Acids with PMO in Adult mdx Mice
(A) Different concentrations of nutrients used for the study. (B–E) Dystrophin expression following single intramuscular injection of 2 μg of PMO into mdx TA muscles (B and C) or intravenous injection of PMO at 25 mg/kg/week for 3 weeks in different amino acid solutions or saline in mdx mice, respectively (D and E). (B) Immunohistochemistry and quantitative analysis for dystrophin-positive fibers in mdx TA muscles treated with single intramuscular injection of 2 μg of PMO in different nutrients (scale bar, 100 μm). The concentration was 5%, as illustrated in (A). For glycine and saline groups, the number of animals used per group is six (n = 6) and the rest is three (n = 3; one-way ANOVA and post hoc Student-Newman-Keuls test). White staining on fiber membrane shows dystrophin expression. (C) Representative western blot and quantitative analysis for dystrophin expression in TA muscles from mdx mice treated with single intramuscular injection of PMO in different solutions. For glycine and saline groups, the number of animals used per group is six (n = 6) and the rest is three (n = 3; one-way ANOVA and post hoc Student-Newman-Keuls test). α-Actinin was used as the loading control. 5, 2.5, and 0.5 μg of total protein from C57BL/6 mice and 50 μg from muscle samples from untreated and treated mdx mice were loaded. TA muscles from C57BL/6 mice were used as normal controls (the same is true for all western blots unless otherwise specified). (D) Immunohistochemistry for dystrophin expression in body-wide muscles from mdx mice treated intravenously with PMO in saline or amino acid solutions (5%) at 25 mg/kg/week for 3 weeks. TA, tibialis anterior. Scale bar, 100 μm. (E) Western blot and quantitative analysis of dystrophin expression in body-wide muscles from mdx mice treated intravenously with PMO in glycine (n = 4), serine, or saline (n = 3) at 25 mg/kg/week for 3 weeks (one-way ANOVA and post hoc Student-Newman-Keuls test). 0.5, 2.5, and 5 μg of total protein from C57BL/6 mice and 50 μg from muscle samples from untreated and treated mdx mice were loaded. (F) Immunohistochemistry for dystrophin expression in body-wide muscles from mdx mice treated intravenously with PMO in glycine at 25 mg/kg/week for 3 weeks with glycine (+Gly) or without additional glycine (−Gly) every other day for 5 weeks (scale bar, 100 μm). (G) Western blot for dystrophin expression in body-wide muscles from mdx mice treated intravenously with PMO in glycine at 25 mg/kg/week for 3 weeks with glycine (+Gly) or without additional glycine (−Gly) every other day for 5 weeks. 0.5, 2.5, 5, and 15 μg of total protein from C57BL/6 mice and 50 μg from muscle samples from untreated and treated mdx mice were loaded. TA, tibialis anterior; Q, quadriceps; G, gastrocnemius; T, triceps; A, abdominal muscle; D, diaphragm. (H) Quantitative analysis of dystrophin expression in body-wide muscles from mdx mice treated intravenously with PMO in glycine at 25 mg/kg/week for 3 weeks with glycine (+Gly) or without additional glycine (−Gly) every other day for 5 weeks (n = 4; one-way ANOVA and post hoc Student-Newman-Keuls test). (I) ELISA assay for measurement of glycine in serum from mdx mice treated intravenously with PMO in glycine at 25 mg/kg/week for 3 weeks with glycine (+Gly) or without additional glycine (−Gly) every other day for 5 weeks (n = 4). Data are presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.001.