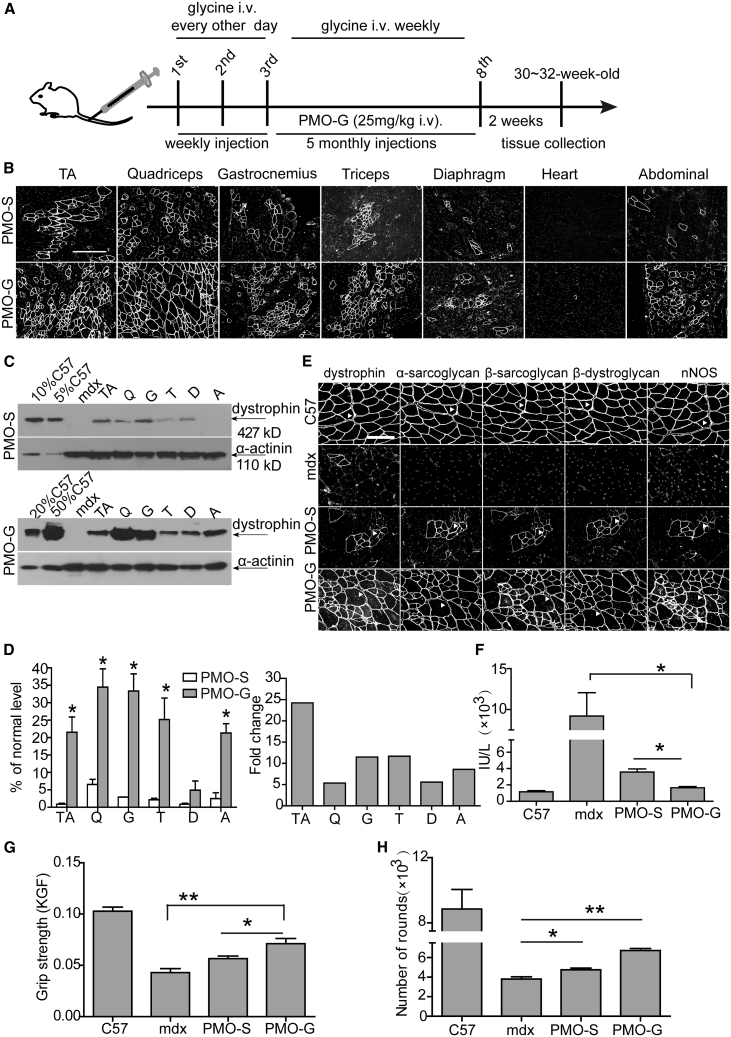
Figure 2.
Long-Term Repeated Administrations of PMO in Glycine (PMO-G) or in Saline (PMO-S) in Adult mdx Mice
PMO-G was administered intravenously into adult mdx mice at 25 mg/kg/week for 3 weeks with additional glycine administration every other day intravenously followed by 25 mg/kg/month for 5 months with additional glycine administration every week intravenously. (A) Diagram of dosing regimen for the long-term study in mdx mice. i.v., intravenous injection. (B) Immunohistochemistry for dystrophin expression in body-wide muscles from mdx mice treated with PMO-S or PMO-G (scale bar, 100 μm). (C) Western blot for dystrophin expression in body-wide muscles from mdx mice treated with PMO-G or PMO-S. 2.5, 5, 10, and 25 μg of total protein from C57BL/6 mice and 50 μg from muscle samples from untreated and treated mdx mice were loaded. TA, tibialis anterior; Q, quadriceps; G, gastrocnemius; T, triceps; A, abdominal muscle; D, diaphragm. (D) Quantitative analysis of dystrophin expression in body-wide muscles from treated mdx mice (n = 4; two-tailed t test). (E) Re-localization of DAPC components in treated mdx mice to assess dystrophin function and recovery of normal myoarchitecture (scale bar, 50 μm). The arrowheads point to identical myofibers. (F) Measurement of serum creatine kinase (CK) levels in treated mdx mice (n = 4; one-way ANOVA and post hoc Student-Newman-Keuls test). (G) Muscle function was assessed to determine the physical improvement with grip strength test (n = 4; one-way ANOVA and post hoc Student-Newman-Keuls test). (H) Measurement of muscle endurance with the running wheel test (n = 4; one-way ANOVA and post hoc Student-Newman-Keuls test). Data are presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.001.