Abstract
Purpose:
To investigate the utility of magnetic resonance elastography (MRE) vs. ultrasound (US) point shear wave elastography (pSWE) for the assessment of chronic renal allograft dysfunction, prediction of outcome and determine the correlation with Banff pathology scores.
Methods:
In this IRB approved prospective study, 27 enrolled patients with functional (n=15) and chronic dysfunctional (n=12) renal allografts underwent same day 2D MRE and pSWE. Histogram parameters [including mean, median, standard deviation, kurtosis and skewness] of the magnitude of the complex shear modulus (MRE) and median Young’s modulus (pSWE) were measured in the cortex (MRE and pSWE) and combined corticomedullary regions (MRE). Histopathology was available for 16 patients (4 functional, 12 dysfunctional).
Results:
MRE and pSWE stiffness were not significantly different between functional and dysfunctional groups (p range 0.139–0.347). The skewness of MRE corticomedullary stiffness was significantly lower (p=0.04) in patients with chronic dysfunction and correlated significantly with Banff histopathologic scores (range r=−0.518–0.567, p=0.035–0.040). MRE cortical and corticomedullary mean stiffness showed strong performance in predicting graft loss/relist (AUC 0.958, p=0.011 for both). Reliable pSWE measurements were obtained in 13 patients (48%). pSWE stiffness did not correlate with Banff scores and did not predict outcome.
Conclusions:
The skewness of MRE corticomedullary stiffness is sensitive to changes in chronic allograft dysfunction, while mean/median MRE renal stiffness and median US stiffness did not differentiate patients with stable function vs those with chronic renal allograft dysfunction. MRE corticomedullary mean stiffness appears to be a predictor of graft loss/relist. pSWE was not found to be a useful method for assessing renal allografts.
Keywords: Elasticity Imaging Techniques, Allograft, Fibrosis, Dysfunction
INTRODUCTION
Renal transplantation is the most effective treatment of end-stage renal disease. However, despite improvements in allograft survival [1], renal allograft loss remains an important clinical problem [2]. Detection of renal fibrosis in renal allograft recipients is essential in order to tailor treatment and ensure survival of the allograft. A combination of biopsy, urinalysis and blood markers are currently used to monitor renal transplant health [3]. Biopsy is considered the reference standard by which clinicians can obtain diagnostic and prognostic information in renal allografts [4], however it is an invasive method and is prone to sampling error, and therefore its use as a surveillance tool is an area of debate [5].
Renal allograft fibrosis is a common occurrence in allograft pathology [6], and is often the final common pathway from a variety of allograft injuries. Because allograft fibrosis is associated with poor renal outcomes [7], there is a need for non-invasive methods to diagnose and quantify the degree of renal fibrosis. Elastography is a technique that enables the non-invasive quantification of mechanical properties by analyzing shear wave propagation through the tissue of interest and enables interrogation of the entire organ. MR elastography (MRE) relies on external equipment to generate shear waves in the tissue of interest whereas ultrasound (US) elastography methods, such as point shear wave elastography (pSWE), utilize focused US beams to compress tissue and generate shear waves. Transient elastography, the most widely available US elastography method, is less suitable for use in renal allograft examination as there is no B-mode imaging capability integrated in the unit. Fibrosis is associated with an increased tissue stiffness due to factors such as collagen deposition and so elastography has become the imaging reference standard method to stage fibrosis in the liver [8]. More recently MR [9–12] and US-based [13–16] elastography approaches have been used to study renal transplants with varying results. There is conflicting published data, with studies showing increased stiffness in dysfunctional allografts using MR and US elastography [9, 14, 16], or decreased stiffness in dysfunctional allografts [12], or no change in dysfunctional allografts using both methods [11, 13]. The only previous study to evaluate MR and US elastography in the same population (n=25) reported a reduction in stiffness in dysfunctional allografts with both modalities [17].
In this study, we investigate the utility of MRE vs. pSWE for the assessment of chronic renal allograft dysfunction, prediction of outcome and determine the correlation with Banff pathology scores.
MATERIALS AND METHODS
Patients
This single center prospective study was compliant with the Health Insurance Portability and Accountability Act and approved by the local institutional review board. Between February 2016 and February 2018, written consent was obtained from 27 patients visiting the Recanati/Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, who had received renal allografts, including 15 with functional allografts (M/F 9/6 mean age 55.9±10.7 years, mean eGFR 71.1±15.9 ml/min/1.73m2, time since transplant 1.3±0.7 years) and 12 with chronic dysfunction and fibrosis (M/F 6/6, mean age 50.0±13.0 years, mean eGFR 30.1±15.3 ml/min/1.73 m2, time since transplant 6.5±6.1 years). Patients were classified as having functional or dysfunctional allografts based on serum creatinine (sCr) and eGFR. Functional patients were defined as having a stable eGFR > 45 ml/min/1.73m2 with <25% change in sCr and/or eGFR over the preceding 3 months. Patients were considered to have allograft dysfunction if there was a ≥25% increase in sCr/decrease in eGFR over a period >3 months. Of these 27 patients, 16 (12/12 chronically dysfunctional patients and 4/15 patients with functional allografts) had clinically indicated biopsy performed within 1 year of imaging (mean duration between biopsy and imaging 183±71 days). Patient characteristics are displayed in Table 1.
Table 1:
Patient characteristics for functional and chronic dysfunctional groups.
| Functional (n=15) | Chronic dysfunction (n=12) | P | |
|---|---|---|---|
| Gender (M/F) | 9/6 | 6/6 | 0.707a |
| Mean age (years) | 55.9±10.7 (28–67) | 50.0±13.0 (27–69) | 0.236b |
| BMI | 27.8±4.7 (19.7–33.9) | 31.2±6.6 (23.9–45.2) | 0.277b |
| Serum creatinine (mg/dl) | 1.05±0.22 (0.68–1.46) | 2.35±0.99 (1.25–5.02) | <0.001b |
| eGFR (ml/min/1.73m2) | 71.1±15.9 (50.1–107.9) | 30.1±15.3 (11.3–68.3) | <0.001b |
| Blood Urea Nitrogen (mg/dl) | 21.1±3.8 (18–30) | 41.0±12.9 (26–60) | <0.001b |
| Biopsy | 4/15 | 12/12 | <0.001a |
| Race/ethnicity | |||
| African American | 3 | 6 | 0.194a |
| Caucasian | 5 | 3 | >0.99a |
| Other | 7 | 3 | 0.088a |
| Biopsy to imaging interval (d) | 281±59 (209–344) | 151±37 (94–224) | <0.001b |
| Time since Tx (y) | 1.34±0.71 (0.55–2.53) | 6.51±6.14 (1.52–22.0) | <0.001b |
| Deceased donor (%) | 40% | 83% | 0.033a |
| Banff scores | |||
| ci+ct | 0.5±0.6 | 4.3±1.0 | 0.001b |
| cv+cg | 0±0 | 1.5±1.3 | 0.03b |
| i+t | 0±0 | 1.4±1.6 | 0.103b |
| iIFTA | 0±0 | 2.3±0.8 | 0.001b |
| Banff chronicity | 0.5±0.6 | 5.8±1.5 | 0.001b |
| ah | 0±0 | 2.2±1.3 | 0.013b |
| g | 0±0 | 0.8±1.0 | 0.170b |
| v | 0±0 | 0±0 | >0.99b |
| ptc | 0±0 | 1.0±1.0 | 0.379b |
eGFR = estimated glomerular filtration rate; BMI = body mass index; ci = interstitial fibrosis; ct = tubular atrophy; cv = vascular fibrous intimal thickening; cg = glomerular double contours; i = inflammation; t = tubulitis; iIFTA = interstitial fibrosis and tubular atrophy in areas of inflammation; ah = arteriolar hyalinosis; g = glomerulitis; v = intimal arteritis; ptc = peritubular capillaritis
Fisher’s Exact test
Mann-Whitney U test
MRI Acquisition
MRI was performed on a 1.5T system (Aera, Siemens Healthineers, Erlangen, Germany) using an 18 channel flexible coil and a 32 channel integrated spine coil. Subjects were instructed to fast for 4 hours prior to imaging.
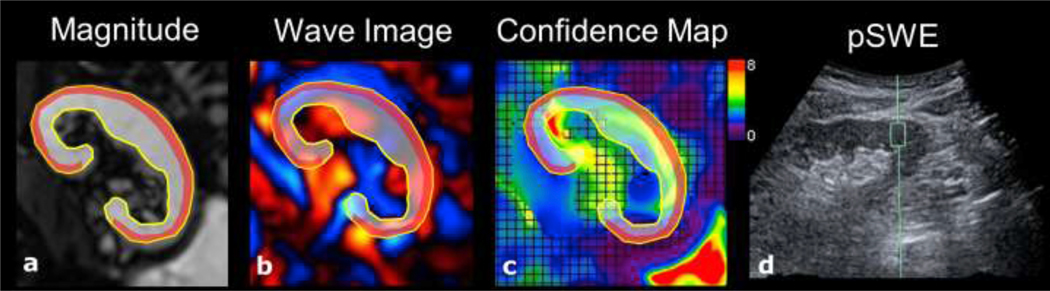
After axial and coronal T2-weighted anatomical imaging (HASTE), 2D MRE data were acquired using a prototype spin-echo echo planar imaging (SE-EPI) sequence with motion encoding gradients applied in the slice-select direction. A 19 cm plastic paddle was placed anteriorly over the allograft and connected to an active pneumatic driver located outside the scan room. Ten slices were acquired in a true coronal orientation through the hilum of the allograft, with a repetition time of 1500ms, time to echo of 48ms, acquisition matrix 256×256, field of view 360–400 mm2, slice thickness 3mm, slice gap 1.5mm, GRAPPA parallel imaging factor of 2, vibration frequency 60Hz, bandwidth 1502 Hz/px, 4 offsets of the wave propagation obtained in the through plane direction, and acquisition time of 17s. 2D MRE data were reconstructed inline using a commercially available 2D multi-model direct inversion (MMDI) algorithm [18]. The algorithm produced magnitude, phase, colorized wave propagation and elastogram images. A confidence mask was also produced which, when overlaid on the elastogram, highlighted regions of reliable measurement (Figure 1). In addition to MRE and HASTE sequences, the MRI protocol also included the following standard sequences: axial T1-weighted VIBE, 3D in- and out-of-phase and diffusion-weighted imaging. Dynamic contrast enhanced MRI was performed in 10 patients with eGFR>30 ml/min/1.73m2, but data was not analyzed as part of this study.
Figure 1:
28-year-old male with functional renal allograft (eGFR 71.8 ml/min/1.73m2). The corticomedullary ROI is outlined is shaded grey, outlined in yellow; and cortical ROI is shaded red. Images depict a) MRE magnitude, b) MRE wave propagation, c) MRE stiffness map with overlaid confidence map signifying areas of reliable measurement, and d) pSWE measurement. MRE measured a corticomedullary stiffness of 4.26 kPa and cortical stiffness of 2.81 kPa. pSWE cortical stiffness was 13.0 kPa.
MRE Analysis
MRE data were analyzed using ImageJ software [19] by an MR physicist with 4 years of experience (--). Freehand ROIs were drawn in the cortical (for comparison with pathology) and combined corticomedullary regions on MRE magnitude images with anatomical imaging referenced for positioning. Cortical ROIs encompassed the cortex at the periphery of the allograft while corticomedullary ROIs encompassed the cortex and medulla, with care taken to exclude the renal sinus. ROIs were drawn in each slice with edge slices discarded to avoid partial volume effects. Measurements were considered reliable in areas with a confidence threshold ≥90%. Mean and median stiffness was determined weighted by ROI size. Histogram parameters skewness, kurtosis and standard deviation were also measured over the ROI using ImageJ. The stiffness is presented as the magnitude of the complex shear modulus |G*| in units of kPa.
pSWE US
Immediately before/after the MRI, patients underwent a pSWE US exam, on a Siemens Acuson S2000 or Acuson S3000 clinical ultrasound system (Virtual Touch Quantification, Siemens, Erlangen, Germany) using a curved transducer (6C1 HD). pSWE capability was identical on both systems. pSWE measurements were acquired by one of three experienced radiologists (-- with 8 years’ experience, -- with 16 years’ experience and -- with 4 years’ experience respectively in abdominal ultrasound), each of whom were blinded to patient’s clinical and pathological data. With the patient in the supine position, the allograft was initially examined with B-mode imaging to assess for hydronephrosis, which was not present in our cohort. Then, a fixed ROI (0.5 × 1.0 cm) was positioned in the renal cortex and 10 valid measurements were performed in each of the upper, middle and lower poles of the renal allograft (Figure 1). Measurements were limited to a depth of 7 cm. In each pole the measurements were acquired from the same location to limit the inter-quartile range (IQR) of the measurements. Care was taken to exclude renal medulla and sinus from the ROI as much as possible. Patients were instructed to suspend breathing during each measurement.
pSWE Analysis
pSWE values are presented as the median of 30 successful measurements, consisting of 10 measurements in each pole. In cases where 30 successful measurements were not possible due to measurement failure, the median of the successful measurements is reported. pSWE values are acquired as shear wave speed (m/s) and converted to Young’s Modulus (E) via the relation E= wave speed2 x ρ, with ρ representing the density of tissue and assumed to be that of water (1000kg/m3). pSWE measurements were considered valid if the interquartile-range (IQR) was <30% of the median value [20] and success rate (percentage of successful measurements obtained) was >60% [21].
Pathology
All patients with chronically dysfunctional allografts and 4/15 patients with functional allografts underwent clinically indicated US guided biopsy prior to the MRI examination, with an average interval of 183±71 days (range 94–344 days). Biopsy samples were analyzed by a renal pathologist with 7 years’ experience (--). Banff scores for inflammation (i), tubulitis (t), glomerulitis (g), arteriolar hyalinosis (ah), intimal arteritis (v), interstitial fibrosis (ci), tubular atrophy (ct), vascular fibrous intimal thickening (cv), glomerular double contours (cg), peritubular capillaritis (ptc) and interstitial fibrosis and tubular atrophy in areas of inflammation (i-IFTA) were recorded based on the Banff 2015 classification [22]. Pathology parameters were grouped into those representing inflammation and tubulitis (i+t), interstitial fibrosis and tubular atrophy (ci+ct), glomerular and vascular change (cv+cg) and a combined Banff chronicity score (ci+ct+cv+cg).
Statistical Analysis
Quantitative variables are presented as mean ± standard deviation. Statistical difference between parameters in patients with chronically dysfunctional and stable allografts was assessed using Mann-Whitney U tests. Difference in categorical variables were determined using Fisher’s Exact test. Between pole pSWE measurement differences were assessed using a Kruskal-Wallis test. Correlations between each of MRI/US and pathology parameters were assessed using Spearman correlation analysis. Backward elimination multiple regression analysis was used for multivariate analysis of factors affecting MRE and pSWE stiffness measurement, with variables previously shown to impact MRE and pSWE stiffness measurements included (body mass index (BMI), age, gender, time since transplant, pSWE measurement depth). ROC analysis was performed to assess the predictive value of elastography parameters for determining graft loss or relist outcomes on follow up. A two-tailed p-value less than 0.05 was considered to be significant. Statistical analyses were performed using SPSS software (Version 20, Chicago, IL).
RESULTS
Technical success of MRE and pSWE
MRE stiffness measurements were not considered reliable in the cortical region of two patients due to a combination of attenuation of the shear waves by subcutaneous and intrabdominal fat layers and thin cortical regions. pSWE measurements were not obtained in two patients due to an allograft depth > 7 cm. In the remaining patients, 15/25 (60%) pSWE exams had IQR<30% of the median value and 22/25 (88%) exams had a success rate >60%. This resulted in only 13/25 (52%) valid pSWE exams. Examples of MRE and pSWE images are shown in Figure 1.
Functional vs chronically dysfunctional allografts
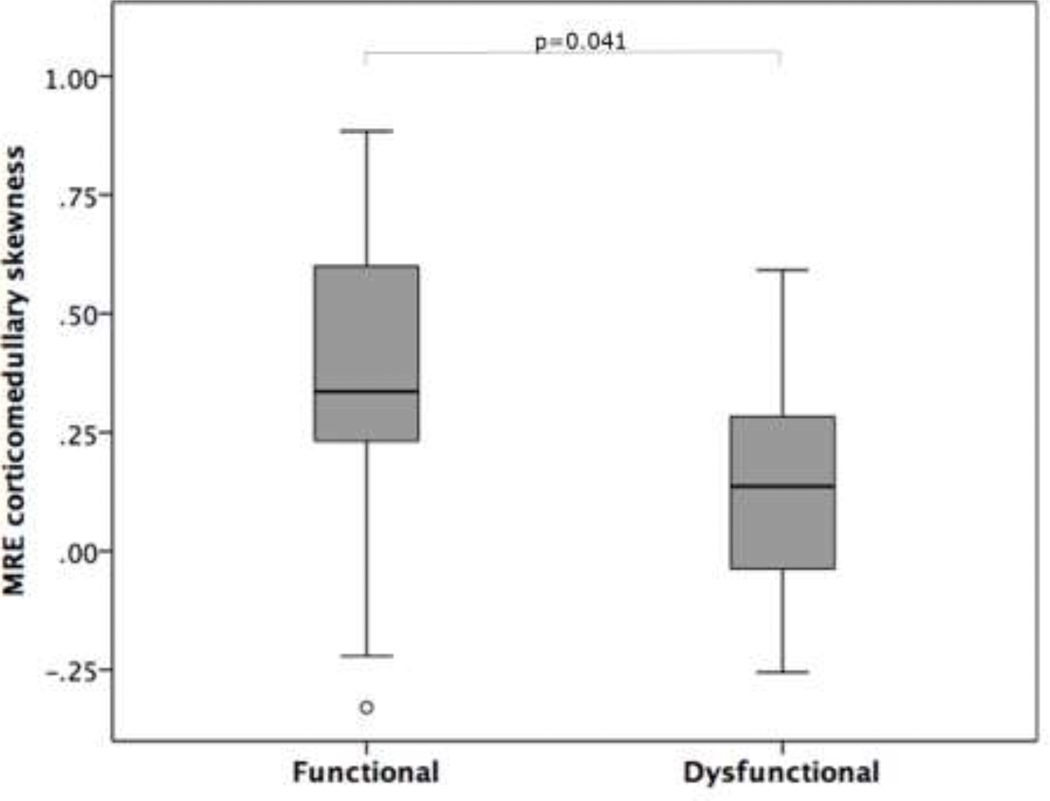
There was no significant difference in mean and median MRE stiffness measurements of the cortical and corticomedullary regions between stable and dysfunctional allografts (Table 2). No significant correlation was observed between MRE stiffness measurements and eGFR. Examination of MRE stiffness histogram parameters revealed a significantly lower corticomedullary skewness in dysfunctional vs stable allografts (0.13±0.23 vs 0.37±0.34, p=0.041, Figure 2), while kurtosis and SD parameters showed no significant difference between the groups. Backward elimination multiple regression analysis showed age, gender, BMI and time since transplant had no effect on MRE corticomedullary stiffness (p=0.073–0.341).
Table 2:
MRE and pSWE stiffness measurements in patients with functional and dysfunctional allografts.
| Functional (n=15) | Chronic dysfunction (n=12) | p* | |
|---|---|---|---|
| Mean MRE corticomedullary stiffness | 3.24±0.69 kPa | 3.73±0.95 kPa | 0.300 |
| Mean MRE cortical stiffness | 2.43±0.61 kPa | 2.84±0.74 kPa | 0.139 |
| Skewness of MRE corticomedullary stiffness | 0.37±0.34 | 0.13±0.23 | 0.041 |
| pSWE stiffness | 13.22±4.64 kPa | 13.46±3.84 kPa | 0.647 |
Mann-Whitney U test
Figure 2:
Boxplot depicting significantly more positive skewness of MRE corticomedullary stiffness in functioning allografts compared to dysfunctional allografts.
No significant difference was observed in Young’s Modulus, E, between functional and dysfunctional groups when including all measurements (N=27, 13.22±4.64 vs 13.46±3.84 kPa, p=0.89) and only reliable measurements (N=13, 13.19±2.65 vs 14.09±4.58 kPa, p=0.681).
There was no significant difference between the stiffness of upper, middle and lower renal poles as measured by reliable pSWE acquisitions (p=0.252). pSWE measurement IQR was significantly negatively correlated with stiffness (r=−0.564, p=0.045). Cortical MRE and pSWE stiffness measurements were not significantly correlated (p=0.544). Backward elimination multiple regression analysis found only BMI to be significantly associated with pSWE stiffness (p=0.004). Representative images from a functional allograft, dysfunctional allograft and dysfunctional allograft requiring hemodialysis following the study are shown in Figure 3.
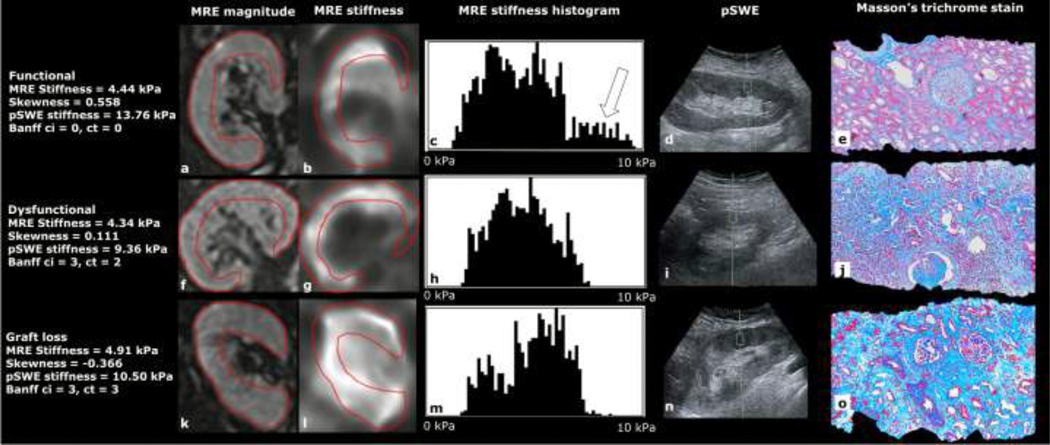
Figure 3:
Example images from a patient with a functional allograft (top, 55-year-old female, eGFR 78.4 ml/min/1.73m2), dysfunctional allograft (middle, 42-year-old male, eGFR 23.4 ml/min/1.73m2) and a patient who was referred for hemodialysis 188 days following the study exam (bottom, 54-year-old male, eGFR 11.3 ml/min/1.73m2), showing MRE magnitude, MRE stiffness, MRE stiffness histogram, pSWE acquisition and Masson’s trichrome stained biopsy slides. More positive skewness is seen in the functional allograft (c, white arrow) compared to the dysfunctional allografts (h, m). The mean stiffness over the corticomedullary ROI (red) is almost identical in the functional and dysfunctional allografts (4.44 kPa and 4.34 kPa) but it is elevated in the allograft in the patient requiring hemodialysis (4.91 kPa). Significant areas of fibrosis (blue stain) are present on Masson’s trichrome biopsy samples from dysfunctional allografts (j, o) compared to the functional allograft (e).
Correlation with pathology
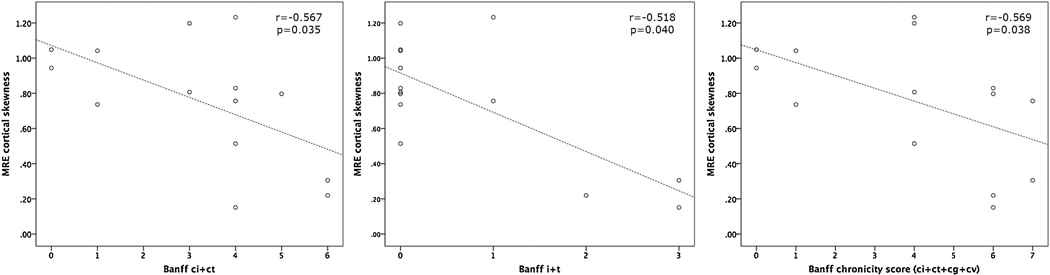
Mean and median cortical MRE stiffness did not significantly correlate with Banff pathology scores. However, the skewness of cortical MRE stiffness significantly correlated with ci+ct (r=−0.567, p=0.035), i+t (r=−0.518, p=0.040) and Banff chronicity (r=−0.559, p=0.038) as shown in Figure 4. pSWE stiffness did not correlate with Banff pathology scores (p=0.245−0.369).
Figure 4:
Scatter plots displaying significant relationships between the skewness of MRE cortical stiffness and Banff markers of interstitial fibrosis and tubular atrophy [scores ci+ct (left)], inflammation and tubulitis [i+t (middle)] and a combined Banff chronicity score (ci+ct+cg+cv, right) in 14 patients. These data suggest patients with higher Banff pathology scores exhibit less positive or negative skewness within the cortical ROI.
Prediction of outcomes
Patients were followed for a mean duration of 542±245 days (range 192–997 days) following initial imaging. In that time period, a total of three patients from the chronic allograft dysfunction group experienced graft loss or were relisted: two patients were referred for hemodialysis and another patient was relisted for transplant due to eGFR <20 ml/min/1.73m2. No stable function patients experienced graft loss during the study follow-up period. Patients who experienced graft loss or were relisted had significantly higher MRE mean stiffness than patients who did not experience progressive decline in allograft function over the corticomedullary (p=0.011) and cortical (p=0.011) ROIs (Table 3). ROC analysis showed both mean cortical and corticomedullary MRE stiffness had strong diagnostic accuracy for predicting graft loss/relist (AUC=0.958, p=0.011 for both), while pathology variables i+t (AUC 0.615), ci+ct (AUC 0.628), cg+cv (AUC 0.603) and Banff chronicity score (AUC 0.679) were not significant predictors of graft loss/relist (p=0.346–0.501).
Table 3:
MRE measurement of mean stiffness (mean±SD) in patients who experienced graft loss or relist eligibility compared to patients with no progressive decline in kidney function on follow-up.
| Graft loss/relist (n=3) | No progressive decline (n=24) | p* | |
|---|---|---|---|
| Mean cortical stiffness | 3.67±0.42 kPa | 2.48±0.60 kPa | 0.011 |
| Mean corticomedullary stiffness | 4.82±0.63 kPa | 3.29±0.69 kPa | 0.011 |
Mann-Whitney U test
DISCUSSION
In this prospective study, we investigated the ability of MRE and pSWE to differentiate patients with functional vs. those with chronically dysfunctional renal allografts. Our results indicate that the mean and median MRE and median pSWE measurements did not separate functional from dysfunctional allografts. However, MRE skewness appeared sensitive to changes inherent to renal allograft dysfunction. Mean cortical and corticomedullary MRE stiffness showed strong performance in predicting graft loss/relist (AUC of 0.958 for both), albeit in a small sample. These results suggest that MRE is promising for the assessment of outcome in patients with renal allograft dysfunction. pSWE was not found to be a useful technique for differentiating patients with functional and dysfunctional allografts, with a high proportion of pSWE measures found to be unreliable in this cohort.
The existing literature on elastography methods in renal allograft fibrosis is conflicting, with several pSWE studies (cohort range 30–91 patients) and one MRE study (11 patients) reporting similar findings to ours, i.e. no significant difference in mean stiffness between stable and chronically dysfunctional allografts [11, 13, 21, 23]. In contrast, several pSWE studies (cohort range 8–102 patients) have concluded that renal allograft stiffness increases in dysfunctional patients [14, 16, 24] with one study (95 patients) noting an increase in stiffness in allograft patients diagnosed with subclinical rejection [25]. Multiple studies (cohort range 18–164 patients) have also reported a positive correlation between allograft stiffness and Banff scores using pSWE [26, 27], 2D-SWE [20] and TE [28–30]. The same positive association between allograft stiffness and Banff score has been reported in a study of 16 patients utilizing MRE [9]. Contradictory findings have also been published, with a recent studies (22 patients) reporting a decrease in renal stiffness in dysfunctional allografts compared to functional allografts as measured by MRE [12]. In another study from the same group, both MRE and 2D-SWE stiffness was found to be reduced in dysfunctional allografts [17]. These studies utilized different MRE and SWE techniques (3D MRE and 2D-SWE as compared to 2D MRE and pSWE in our study) which may explain the contradictory findings. The dysfunctional cohort also included patients all undergoing hemodialysis which was not the case in our study. In addition to these studies, pSWE studies focused on native dysfunctional kidneys have reported a decreased shear wave speed, and hence stiffness, with decreasing eGFR in 319 patients [31] and decreasing shear wave speed with increasing chronic kidney disease stage in 64 patients [32].
Faced with mixed results in previous studies, interpretation of our results is challenging. Previous studies did not report histogram parameters such as skewness in their analyses. We found allograft corticomedullary skewness was more positively skewed in stable functioning patients compared to dysfunctional patients. Skewness relates to the distribution of pixel values within an ROI. A positive skewness indicates there is a tail of higher stiffness values skewing the distribution to the left of the histogram. Our data reveal that in functional allograft patients the histogram of the corticomedullary ROI included a significantly more positive skew indicating the presence of higher stiffness values within the ROI. The lack of higher stiffness areas in the dysfunctional group may be due to changes in renal perfusion. The kidney is a highly perfused organ and there is evidence to suggest renal stiffness is related to renal hemodynamics [33, 34]. In a porcine model of native kidneys using MRE, acute decreases in renal blood flow were accompanied by reductions in cortical stiffness, with medullary stiffness also significantly decreased at 100% decrease in renal blood flow [35]. The lack of a significant difference in mean stiffness between functional and dysfunctional groups may be also due to the small sample size.
Despite finding no difference in mean stiffness values between functional and dysfunctional groups, we do report significantly higher baseline stiffness in patients who experienced graft loss or relist compared to patients with no progressive decline in allograft function during the study follow-up period. This suggests that changes in stiffness may not be apparent until the more severe stages of kidney dysfunction and that MRE could be used as a tool to identify patients that are at highest risk of graft loss. The findings of increased stiffness in patients who experienced graft loss appear to conflict with the findings of less positive skewness (i.e. lack of a tail of high stiffness values in the histogram) in the dysfunctional group. This conflict may be once again explained by changes in renal perfusion which was not assessed. The sample of patients who experienced graft loss/relist was also small which may bias results.
The lack of consensus on the ability of elastography to detect changes in renal allograft fibrosis highlights the complexity of the measurement. In comparison to the liver, the kidney is an organ with highly anisotropic structures. Diffusion-weighted imaging methods that probe tissue structure have shown that renal cortex and medulla display high fractional anisotropy (FA) values [36]. The effect of renal anisotropy on pSWE measurements was illustrated by Gennisson et al who found that shear wave speed of in-vivo porcine kidneys was dependent on the axis of measurement in relation to the main renal pyramid axis [37]. Furthermore, operator related variables may also effect measured renal stiffness, with Syversveen et al demonstrating that pSWE measurement was significantly affected by the force applied on the transducer during measurement [38]. In the present study, the operators were instructed to exert the minimum force possible on the transducer during pSWE exams, however in cases of deep lying allografts varying degrees of force were necessary to ensure successful measurement of the renal cortex, particularly in the upper and lower poles. Despite these considerations during pSWE exams, a high proportion of measurements were found to be unreliable. An association between subject BMI and measured pSWE stiffness was found in our cohort. Although not previously reported in allografts, researchers have previously noted a correlation between BMI and pSWE stiffness in the pancreas [39] and found unreliable measures to be associated with BMI in the liver [40]. Thus, a variety of physiologic and technical factors can influence renal stiffness measurements.
Our study has several limitations. The first is the small sample size, with only 27 subjects included in analysis. The small sample size means results predicting outcome in 3 patients must be considered with caution. The second issue is that we do not perform protocol biopsies at our institution. All biopsies for this study were performed based on clinical indication, which reflects the practice standard in our institution. The third issue is the use of 2D MRE for analysis. In 2D MRE, only the wave propagation in the through plane axis is encoded into the MR phase image. This is commonly used in liver MRE however the complex anisotropic structure of the kidney may cause wave interference in the renal parenchyma and potentially lead to inaccurate stiffness quantification [41]. A solution to this problem is the use of 3D MRE, in which three orthogonal directions of wave propagation are captured and incorporated into the 3D MRE specific reconstructed stiffness map therefore addressing the issue of oblique waves causing incorrect stiffness measurements. Future studies will evaluate 3D MRE for assessment of allograft dysfunction. While all three radiologists who acquired pSWE measurements for this study were trained, there is a risk of inter-observer measurement variability, which was not assessed. The high number of unreliable pSWE measures is a limitation of the study and may be due to the anisotropy of the kidney or interoperator variability. Finally, while renal perfusion is a recognized confounder of MRE measurement, no measurement of renal perfusion [e.g. using arterial spin labelling (ASL)] was assessed in this study.
In conclusion, our results in a small cohort of patients have demonstrated that MRE histogram parameter skewness appears to be sensitive to changes related to chronic allograft dysfunction. Mean MRE cortical and corticomedullary stiffness were found to be significant predictors of graft loss/relist. Mean and median MRE stiffness as well pSWE measurements were not significantly different between patients with functional allografts and chronic allograft dysfunction and for patients with different Banff diagnoses, likely indicating that a variety of concurrent pathophysiologic processes can contribute to stiffness. From our experience, we cannot recommend pSWE as a diagnostic tool for detecting renal allograft dysfunction due to the high proportion of unreliable measures obtained. Further study is warranted to investigate the role of MRE for interrogation of allograft outcomes.
Highlights.
MRE histogram parameters appear sensitive to renal allograft dysfunction
Mean MRE stiffness was a significant predictor of graft loss and relist
pSWE did not differentiate dysfunctional allografts or correlate with pathology
pSWE had a high proportion of unreliable measurements
Acknowledgements:
The authors thank the participants for their time and are grateful to the MR technologists for assistance with imaging. Study funding was provided by Guerbet LLC.
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- E
Young’s modulus (measured with pSWE)
- eGFR
estimated glomerular filtration rate
- |G*|
magnitude of the complex shear modulus (measured with MRE)
- IQR
interquartile range
- MRE
magnetic resonance elastography
- pSWE
point shear wave elastography
- ROC
receiver operator characteristic curve
- ROI
region of interest
- sCR
serum creatinine
- SE-EPI
spin-echo echo planar imaging
- TE
echo time
- TR
repetition time
- US
ultrasound
Footnotes
Credit author statement
Paul Kennedy: Investigation, Formal analysis, Writing - Original Draft & Editing,,
Octavia Bane: Investigation, Formal analysis, Writing - Review & Editing,
Stefanie J Hectors: Investigation, Writing - Review & Editing
Sonja Gordic: Investigation
Mark Berger: Investigation
Veronica Delaney: Conceptualization, Methodology
Fadi Salem: Conceptualization, Methodology, Investigation
Sara Lewis: Conceptualization, Methodology, Writing - Review & Editing
Madhav Menon: Conceptualization, Methodology
Bachir Taouli: Conceptualization, Methodology, Writing - Review & Editing
Conflicts of Interest
Paul Kennedy: None to declare
Octavia Bane: Received salary support from NIDDK grant 1F32DK109591
Stefanie Hectors: None to declare
Sonja Gordic: Received salary support from Swiss National Science Foundation grant P2ZHP3_161691
Mark Berger: None to declare
Veronica Delaney: None to declare
Fadi Salem: None to declare
Sara Lewis: Receives research support from Bayer and the Society of Abdominal Radiology
Madhav Menon: None to declare
Bachir Taouli: Receives research support from Bayer and Guerbet. Guerbet directly funded patient scans for this study. PI on NIDDK R01 DK113272.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D, Improved graft survival after renal transplantation in the United States, 1988 to 1996, N Engl J Med 342(9) (2000) 605–12. [DOI] [PubMed] [Google Scholar]
- [2].Naesens M, Kuypers DR, De Vusser K, Evenepoel P, Claes K, Bammens B, Meijers B, Sprangers B, Pirenne J, Monbaliu D, Jochmans I, Lerut E, The histology of kidney transplant failure: a long-term follow-up study, Transplantation 98(4) (2014) 427–35. [DOI] [PubMed] [Google Scholar]
- [3].Kasiske BL, Vazquez MA, Harmon WE, Brown RS, Danovitch GM, Gaston RS, Roth D, Scandling JD, Singer GG, Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation, J Am Soc Nephrol 11 Suppl 15 (2000) S1–86. [PubMed] [Google Scholar]
- [4].Williams WW, Taheri D, Tolkoff-Rubin N, Colvin RB, Clinical role of the renal transplant biopsy, Nat Rev Nephrol 8(2) (2012) 110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thaunat O, Legendre C, Morelon E, Kreis H, Mamzer-Bruneel M-F, To Biopsy or Not to Biopsy? Should We Screen the Histology of Stable Renal Grafts?, Transplantation 84(6) (2007) 671. [DOI] [PubMed] [Google Scholar]
- [6].Boor P, Floege J, Renal allograft fibrosis: biology and therapeutic targets, Am J Transplant 15(4) (2015) 863–86. [DOI] [PubMed] [Google Scholar]
- [7].Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD, Predicting subsequent decline in kidney allograft function from early surveillance biopsies, Am J Transplant 5(10) (2005) 2464–72. [DOI] [PubMed] [Google Scholar]
- [8].Kennedy P, Wagner M, Castera L, Hong CW, Johnson CL, Sirlin CB, Taouli B, Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions, Radiology 286(3) (2018) 738–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kirpalani A, Hashim E, Leung G, Kim JK, Krizova A, Jothy S, Deeb M, Jiang NN, Glick L, Mnatzakanian G, Yuen DA, Magnetic Resonance Elastography to Assess Fibrosis in Kidney Allografts, CJASN 12(10) (2017) 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim JK, Yuen DA, Leung G, Jothy S, Zaltzman J, Ramesh Prasad GV, Prabhudesai V, Mnatzakanian G, Kirpalani A, Role of Magnetic Resonance Elastography as a Noninvasive Measurement Tool of Fibrosis in a Renal Allograft: A Case Report, Transplant Proc 49(7) (2017) 1555–1559. [DOI] [PubMed] [Google Scholar]
- [11].Lee CU, Glockner JF, Glaser KJ, Yin M, Chen J, Kawashima A, Kim B, Kremers WK, Ehman RL, Gloor JM, MR elastography in renal transplant patients and correlation with renal allograft biopsy: a feasibility study, Academic Radiology 19(7) (2012) 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marticorena Garcia SR, Fischer T, Dürr M, Gültekin E, Braun J, Sack I, Guo J, Multifrequency Magnetic Resonance Elastography for the Assessment of Renal Allograft Function, Investigative Radiology 51(9) (2016) 591–595. [DOI] [PubMed] [Google Scholar]
- [13].Lee J, Oh YT, Joo DJ, Ma BG, Lee A.l., Lee JG, Song SH, Kim SU, Jung DC, Chung YE, Kim YS, Acoustic Radiation Force Impulse Measurement in Renal Transplantation: A Prospective, Longitudinal Study With Protocol Biopsies, Medicine 94(39) (2015) e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].He W-Y, Jin Y-J, Wang W-P, Li C-L, Ji Z-B, Yang C, Tissue elasticity quantification by acoustic radiation force impulse for the assessment of renal allograft function, Ultrasound in Medicine & Biology 40(2) (2014) 322–329. [DOI] [PubMed] [Google Scholar]
- [15].Bom Jun K, Chan Kyo K, Jung Jae P, Non-invasive evaluation of stable renal allograft function using point shear-wave elastography, Br J Radiol (2017) 20170372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ghonge NP, Mohan M, Kashyap V, Jasuja S, Renal Allograft Dysfunction: Evaluation with Shear-wave Sonoelastography, Radiology 288(1) (2018) 146–152. [DOI] [PubMed] [Google Scholar]
- [17].Marticorena Garcia SR, Guo J, Durr M, Denecke T, Hamm B, Sack I, Fischer T, Comparison of ultrasound shear wave elastography with magnetic resonance elastography and renal microvascular flow in the assessment of chronic renal allograft dysfunction, Acta Radiol (2017) 284185117748488. [DOI] [PubMed] [Google Scholar]
- [18].Silva AM, Grimm RC, Glaser KJ, Fu Y, Wu T, Ehman RL, Silva AC, Magnetic resonance elastography: evaluation of new inversion algorithm and quantitative analysis method, Abdom Imaging 40(4) (2015) 810–817. [DOI] [PubMed] [Google Scholar]
- [19].Schneider CA, Rasband WS, Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis, Nat Meth 9(7) (2012) 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grenier N, Poulain S, Lepreux S, Gennisson J-L, Dallaudière B, Lebras Y, Bavu E, Servais A, Meas-Yedid V, Piccoli M, Bachelet T, Tanter M, Merville P, Couzi L, Quantitative elastography of renal transplants using supersonic shear imaging: a pilot study, European Radiology 22(10) (2012) 2138–2146. [DOI] [PubMed] [Google Scholar]
- [21].Syversveen T, Brabrand K, Midtvedt K, Strøm EH, Hartmann A, Jakobsen JA, Berstad AE, Assessment of renal allograft fibrosis by acoustic radiation force impulse quantification--a pilot study, Transpl. Int. 24(1) (2011) 100–105. [DOI] [PubMed] [Google Scholar]
- [22].Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, Nankivell BJ, Colvin RB, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell L, Drachenberg C, Dragun D, de Kort H, Gibson IW, Kraus ES, Lefaucheur C, Legendre C, Liapis H, Muthukumar T, Nickeleit V, Orandi W Park, M. Rabant, P. Randhawa, E.F. Reed, C. Roufosse, S.V. Seshan, B. Sis, H.K. Singh, Schinstock, A. Tambur, A. Zeevi, M. Mengel, The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology, Am J Transplant 17(1) (2017) 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Syversveen T, Midtvedt K, Berstad AE, Brabrand K, Strom EH, Abildgaard A, Tissue elasticity estimated by acoustic radiation force impulse quantification depends on the applied transducer force: an experimental study in kidney transplant patients, Eur Radiol 22(10) (2012) 2130–7.22610533 [Google Scholar]
- [24].Stock KF, Klein BS, Cong MTV, Regenbogen C, Kemmner S, Buttner M, Wagenpfeil S, Matevossian E, Renders L, Heemann U, Kuchle C, ARFI-based tissue elasticity quantification and kidney graft dysfunction: First clinical experiences, Clin Hemorheol Micro 49(1–4) (2011) 527–535. [DOI] [PubMed] [Google Scholar]
- [25].Kim BJ, Kim CK, Park JJ, Non-invasive evaluation of stable renal allograft function using point shear-wave elastography, BJR 91(1081) (2018) 20170372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stock KF, Klein BS, Vo MTC, Sarkar O, Römisch M, Regenbogen C, Büttner M, Schuster T, Matevossian E, Amann K, Clevert DA, Heemann U, Küchle C, ARFI-based tissue elasticity quantification in comparison to histology for the diagnosis of renal transplant fibrosis, Clin Hemorheol Micro 46(2–3) (2010) 139–148. [DOI] [PubMed] [Google Scholar]
- [27].Chiocchini ALC, Sportoletti C, Comai G, Brocchi S, Capelli I, Baraldi O, Bruno P, Conti F, Serra C, Meola M, Zompatori M, La Manna G, Correlation Between Renal Cortical Stiffness and Histological Determinants by Point Shear-Wave Elastography in Patients With Kidney Transplantation, Prog Transplant 27(4) (2017) 346–353. [DOI] [PubMed] [Google Scholar]
- [28].Nakao T, Ushigome H, Nakamura T, Harada S, Koshino K, Suzuki T, Ito T, Nobori S, Yoshimura N, Evaluation of Renal Allograft Fibrosis by Transient Elastography (Fibro Scan), Transpl P 47(3) (2015) 640–643. [DOI] [PubMed] [Google Scholar]
- [29].Sommerer C, Scharf M, Seitz C, Millonig G, Seitz HK, Zeier M, Mueller S, Assessment of renal allograft fibrosis by transient elastography, Transpl Int 26(5) (2013) 545–551. [DOI] [PubMed] [Google Scholar]
- [30].Arndt R, Schmidt S, Loddenkemper C, Grunbaum M, Zidek W, van der Giet M, Westhoff TH, Noninvasive evaluation of renal allograft fibrosis by transient elastography - a pilot study, Transpl Int 23(9) (2010) 871–877. [DOI] [PubMed] [Google Scholar]
- [31].Asano K, Ogata A, Tanaka K, Ide Y, Sankoda A, Kawakita C, Nishikawa M, Ohmori K, Kinomura M, Shimada N, Fukushima M, Acoustic Radiation Force Impulse Elastography of the Kidneys Is Shear Wave Velocity Affected by Tissue Fibrosis or Renal Blood Flow?, Journal of Ultrasound in Medicine 33(5) (2014) 793–801. [DOI] [PubMed] [Google Scholar]
- [32].Guo LH, Xu HX, Fu HJ, Peng A, Zhang YF, Liu LN, Acoustic radiation force impulse imaging for noninvasive evaluation of renal parenchyma elasticity: preliminary findings, PLOS ONE 8(7) (2013) e68925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lanzman RS, Wittsack HJ, Martirosian P, Zgoura P, Bilk P, Kropil P, Schick F, Voiculescu A, Blondin D, Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results, European Radiology 20(6) (2010) 1485–1491. [DOI] [PubMed] [Google Scholar]
- [34].Heusch P, Wittsack HJ, Blondin D, Ljimani A, Nguyen-Quang M, Martirosian P, Zenginli H, Bilk P, Kropil P, Heusner TA, Antoch G, Lanzman RS, Functional Evaluation of Transplanted Kidneys Using Arterial Spin Labeling MRI, Journal of Magnetic Resonance Imaging 40(1) (2014) 84–89. [DOI] [PubMed] [Google Scholar]
- [35].Warner L, Yin M, Glaser KJ, Woollard JA, Carrascal CA, Korsmo MJ, Crane JA, Ehman RL, Lerman LO, Noninvasive in vivo assessment of renal tissue elasticity during graded renal ischemia using MR Elastography, Investigative radiology 46(8) (2011) 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ries M, Jones RA, Basseau F, Moonen CTW, Grenier N, Diffusion tensor MRI of the human kidney, Journal of Magnetic Resonance Imaging 14(1) (2001) 42–49. [DOI] [PubMed] [Google Scholar]
- [37].Gennisson JL, Grenier N, Combe C, Tanter M, Supersonic Shear Wave Elastography of in Vivo Pig Kidney: Influence of Blood Pressure, Urinary Pressure and Tissue Anisotropy, Ultrasound in Medicine and Biology 38(9) (2012) 1559–1567. [DOI] [PubMed] [Google Scholar]
- [38].Syversveen T, Midtvedt K, Berstad AE, Brabrand K, Strøm EH, Abildgaard A, Tissue elasticity estimated by acoustic radiation force impulse quantification depends on the applied transducer force: an experimental study in kidney transplant patients, European Radiology 22(10) (2012) 2130–2137.22610533 [Google Scholar]
- [39].Stumpf S, Jaeger H, Graeter T, Oeztuerk S, Schmidberger J, Haenle MM, Kratzer W, Ulm E-SG, Influence of age, sex, body mass index, alcohol, and smoking on shear wave velocity (p-SWE) of the pancreas, Abdom Radiol 41(7) (2016) 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bota S, Sporea I, Sirli R, Popescu A, Danila M, Jurchis A, Gradinaru-Tascau O, Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of Acoustic Radiation Force Impulse (ARFI) elastography-Analysis of a cohort of 1031 subjects, European Journal of Radiology 83(2) (2014) 268–272. [DOI] [PubMed] [Google Scholar]
- [41].Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL, Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers, J Magn Reson Imaging 41(2) (2015) 369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]