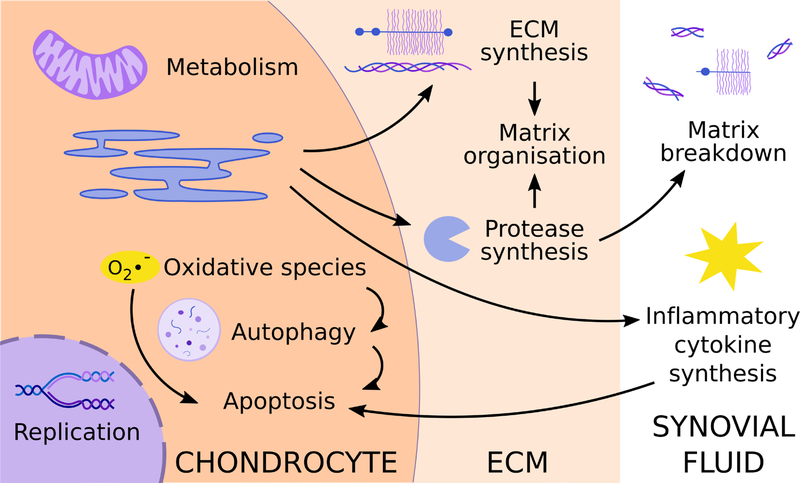
Fig. 1. Biological processes identified as being affected by DEX either in healthy or diseased cartilage in studies using in vivo models, cartilage explants or chondrocyte monoculture.
DEX affects matrix organisation at the level of both ECM and protease synthesis, although studies often disagree on the specific up- or down- regulation of ECM specific components. There is a consensus that, under arthritic stresses, DEX prevents the upregulation of protease synthesis, which can prevent matrix loss. However, at higher doses, in healthy cartilage, DEX may increase the rate of matrix degradation or the organisation of the matrix itself. This could be due to effects on matrix components and proteases or due to intracellular effects on metabolism and the production of ROS that activate autophagy and lead to significant cell death. Data from in vitro studies have suggested that DEX maintains cell viability under arthritic stress, which could be linked to a DEX-induced reduction in inflammatory cytokine synthesis. Alternatively, the metabolic processes that DEX dysregulates in healthy tissue could serve to rescue changes in those processes after the initiation of arthritis. While the induction of autophagy in healthy tissue could lead to chondrocyte death and subsequent matrix breakdown, autophagy is suppressed in arthritic contexts, so DEX could serve to rescue these cellular processes in a diseased state. It remains to be seen whether DEX inhibits proliferation under arthritic stress and what role this might play in disease progression and whether the phenomenon of DEX-induced reduction of proliferation in healthy cartilage is due to cells becoming quiescent or senescent. Each possibility would have a significantly different biological outcome on cartilage exposed to DEX for an extended time.