Figure 1: Effect of the nitro group position and alkyl substitution on the anticlostridial activity of nitroim idazoles and nitrothiazoles.
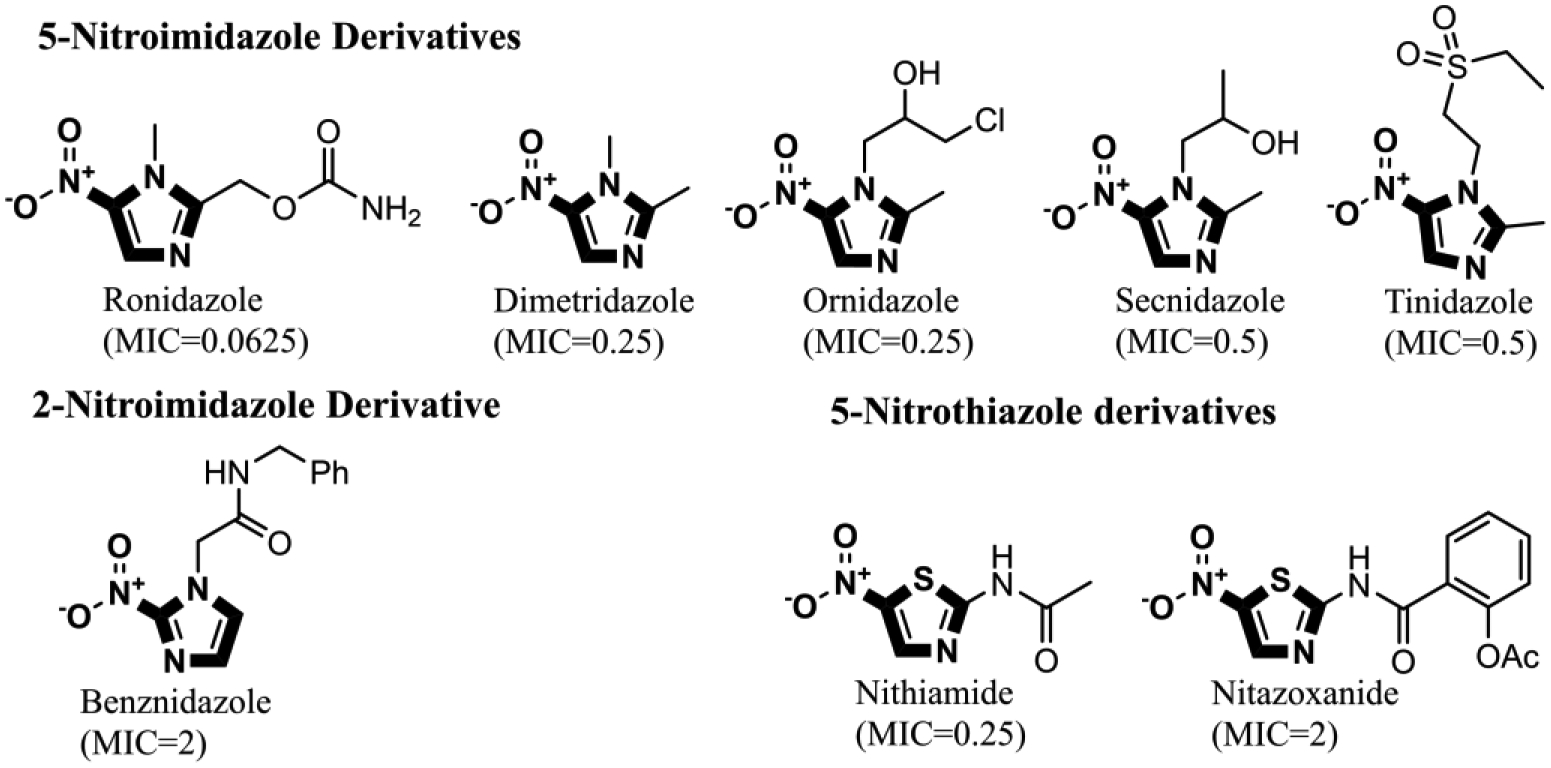
5-nitroimidazoles were found to be more potent than 2-nitroimdazle. Contrarily, variation in the alkyl substitution of 5-nitroimdazole did not significantly affect anticlostridial activity. Additionally, Increasing the size of the substitution at position 2 of the nitrothiazole ring increased the MIC from 0.25 μM in the case of nithiamide to 2 μM in case of nitazoxanide. MIC values are against C. difficile NAP07 and are expressed in μM.
