Abstract
Introduction
The objective of this study was to assess efficacy and safety of repository corticotropin injection (RCI) in subjects with active rheumatoid arthritis (RA) despite treatment with a corticosteroid and one or two disease-modifying antirheumatic drugs (DMARDs).
Methods
All subjects received open-label RCI (80 U) twice weekly for 12 weeks (part 1); only those with low disease activity [LDA; i.e., Disease Activity Score 28 joint count and erythrocyte sedimentation rate (DAS28-ESR) < 3.2] were randomly assigned to receive either RCI (80 U) or placebo twice weekly during the 12-week double-blind period (part 2). The primary efficacy endpoint was the proportion of subjects who achieved LDA at week 12. Secondary efficacy endpoints included proportions of subjects who maintained LDA during weeks 12 through 24 and achieved Clinical Disease Activity Index (CDAI) ≤ 10 at weeks 12 and 24. Safety was assessed via adverse event reports.
Results
Of the 259 enrolled subjects, 235 completed part 1; 154 subjects (n = 77 each for RCI and placebo) entered part 2, and 127 (RCI, n = 71; placebo, n = 56) completed. At week 12, 163 subjects (62.9%) achieved LDA and 169 (65.3%) achieved CDAI ≤ 10 (both p < 0.0001). At week 24, 47 (61.0%) RCI-treated and 32 (42.1%) placebo-treated subjects maintained LDA (p = 0.019); 66 (85.7%) RCI-treated and 50 (65.8%) placebo-treated subjects maintained CDAI ≤ 10 (p = 0.004). No unexpected safety signals were observed.
Conclusions
RCI was effective and generally safe in patients with active RA despite corticosteroid/DMARD therapy. By week 12, > 60% of patients achieved LDA, which was maintained with 12 additional weeks of treatment. Most patients who achieved LDA maintained it for 3 months after RCI discontinuation.
Trial Registration
Clinicaltrials.gov identifier NCT02919761.
Electronic Supplementary Material
The online version of this article (10.1007/s40744-020-00199-3) contains supplementary material, which is available to authorized users.
Keywords: Active disease, Clinical trial, Corticosteroids, Disease-modifying antirheumatic drugs, Low disease activity, Repository corticotropin injection, Rheumatoid arthritis, Withdrawal study
Key Summary Points
| Why carry out this study? |
| Despite the availability of numerous biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs) and despite using glucocorticoids, many patients with rheumatoid arthritis (RA) are unable to achieve or maintain remission or low disease activity (LDA) with these agents and, as a result, may sustain irreversible joint damage. |
| Repository corticotropin injection (RCI) is a naturally sourced complex mixture of adrenocorticotropic hormone analogues and other pituitary peptides that functions as an agonist of all five melanocortin receptors and has several potential mechanistic pathways that may contribute to its therapeutic effects in RA. |
| The current study was undertaken to confirm findings from previous small open-label studies by assessing the efficacy, safety, and tolerability of RCI in a larger population of subjects with active RA despite treatment with prednisone (or an equivalent) and one or two conventional synthetic DMARDs or one biologic DMARD via a randomized, double-blind, placebo-controlled withdrawal trial with an open-label run-in period. |
| What was learned from this study? |
| > 60% of patients achieved LDA during 12 weeks of open-label RCI therapy, which was maintained with 12 additional weeks of RCI maintenance therapy; most patients who achieved LDA maintained it for 3 months after RCI discontinuation. |
| In patients with active RA despite corticosteroid/DMARD therapy, RCI was generally safe and was associated with significant, durable, and beneficial effects on disease activity. |
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease in which proinflammatory cytokines act as mediators of synovial inflammation, with resulting bone and cartilage damage in multiple joints [1, 2]. Although the distribution of RA varies by age and geographic location, in developed countries the estimated incidence and prevalence of RA in adults range from 5 to 50 per 100,000 and 0.5–1.0%, respectively [3]. A treat-to-target approach has been advocated for RA, with the goal being remission or low disease activity (LDA) if remission cannot be obtained [4, 5]. The cornerstones for treatment are conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologic DMARDs (bDMARDs), and targeted synthetic DMARDs, which suppress inflammation that leads to eventual joint damage [6]. If instituted early, effective DMARD therapy may prevent irreversible joint damage and improve function [6].
Rates of remission and clinical response in DMARD-treated patients with RA vary widely depending on the agents used, whether monotherapy or combination therapy is employed, the time point(s) assessed, and the criteria for defining remission and response—whether strict, such as the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) definitions [4, 5], or less stringent, such as Disease Activity Score with 28 joint count and erythrocyte sedimentation rate (DAS28-ESR) < 2.6. Generally, at 1 year, estimates of remission range from 25% with strict metrics to 55% with less stringent metrics, and response rates to therapy range from 35–65% [7]. Thus, despite availability of numerous biologic and nonbiologic DMARDs, substantial proportions of patients are unable to achieve or maintain remission or LDA with these agents and may sustain irreversible joint damage and associated decline in their ability to perform basic physical activity [6].
Corticosteroids are often used to rapidly control inflammation in patients with RA who are initiating or changing DMARD therapy, but their association with numerous adverse events (AEs) limits their use [4, 5, 8]. Of particular concern in patients with RA, corticosteroid use for > 3 months, particularly at a high dose, is associated with rapid, persistent bone loss, which contributes to increased risk of osteoporosis and fractures [1]. Hence, additional treatment options for patients with RA are needed.
Repository corticotropin injection (RCI; Acthar® Gel) is a naturally sourced complex mixture of adrenocorticotropic hormone analogues and other pituitary peptides. As an agonist of all five melanocortin receptors (MCRs), RCI has several potential mechanistic pathways that may contribute to its therapeutic effects in RA [9, 10]. Results from preclinical studies suggest several nonsteroidogenic pathways for RCI that may affect inflammation and immune regulation. Activation of MCR1 has been shown to affect the nuclear factor-κB (NF-κB) pathway, leading to downregulation of inflammatory cytokines [11–13]. Multiple cells of the immune system express MCR1, MCR3, and/or MCR5, suggesting they have additional roles in mediating inflammation [11, 14, 15]. Further, MCR1 and MCR5 are present on human articular chondrocytes and rheumatoid synovial fibroblasts, which are involved in the chronic immune response in RA [11, 16]. All five MCRs are expressed on osteoclasts and osteoblasts [17, 18], a finding that could have implications for the bone resorption associated with RA [11, 14, 15, 18].
The evidence for the clinical effectiveness of RCI in patients with RA was suggested in small open-label studies [19–21]. The current study was undertaken to confirm these findings in a larger population via a randomized, double-blind, placebo-controlled withdrawal trial with an open-label run-in period. Our objective was to assess the efficacy, safety, and tolerability of RCI in subjects with active RA despite treatment with prednisone (or an equivalent) and one or two csDMARDs or one bDMARD.
Methods
Study Design
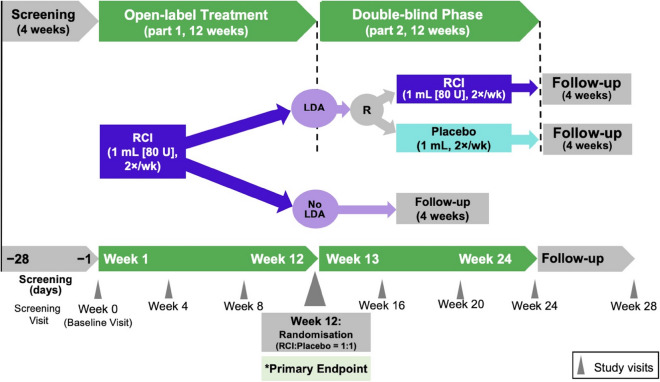
The design of this two-part multicenter, randomized, placebo-controlled withdrawal study is shown in Fig. 1. All enrolled subjects received open-label RCI (1 ml, 80 U) subcutaneously twice weekly for 12 weeks (part 1), a dosage that previous studies suggest is effective [20, 21]. Subjects were then assessed for treatment response using the DAS28-ESR, with thresholds of 2.6, 3.2, and 5.1 suggesting remission, LDA, and high disease activity, respectively [22, 23]. Subjects who achieved LDA (DAS28-ESR < 3.2) at week 12 were randomly assigned to receive either subcutaneous RCI (1 ml, 80 U) or matching placebo (1 ml) twice weekly during the 12-week, double-blind withdrawal period (part 2).
Fig. 1.
Study design. LDA low disease activity, R randomization, RCI repository corticotropin injection
The study was conducted at 60 centers in four countries (see electronic supplementary material for details on study centers) from November 7, 2016, to February 13, 2019, in accordance with the principles and requirements of the Declaration of Helsinki, Good Clinical Practices, and clinical trial registration (clinicaltrials.gov identifier NCT02919761). All investigators obtained institutional review board/independent ethics committee approval. All subjects provided informed consent (including consent for publication) before any study procedures were performed.
Key Study Entry Criteria
Individuals eligible for participation included men and nonpregnant, nonlactating women aged ≥ 18 years who met the 2010 ACR/EULAR criteria [24] for having RA that was active, defined as DAS28-ESR > 3.2, despite treatment with a stable dose (5–10 mg) of prednisone (or equivalent) and one or two csDMARDs or one bDMARD (Table S1 in the electronic supplementary material). Subjects who were taking nonsteroidal anti-inflammatory drugs were required to be on a stable dose for 4 weeks before screening and remain on a stable dose throughout study participation.
Individuals were ineligible for participation if they had any rheumatic autoimmune disease or inflammatory joint disease other than RA, had a history of using adrenocorticotropic hormone (ACTH) preparations for RA or a history of sensitivity to ACTH preparations, or had known contraindications to RCI use; had used any investigational treatment for RA or any biologic investigational agent in the 24 weeks before the first dose of study drug, any nonbiologic investigational agent within 6 weeks before the first dose of study drug, B cell-mediated therapies in the 24 weeks before screening, or intraarticular corticosteroids within 14 days before screening; or had type 1 or type 2 diabetes mellitus, a history of chronic active hepatitis or tuberculosis, a solid tumor or hematologic malignancy, drug/alcohol abuse, or a clinically significant infection.
The use of intraarticular steroids; live or attenuated vaccines; enteral or parenteral immunosuppressive medications; or an investigational drug, device, or procedure administered as part of a research study was not permitted during the trial.
Procedures and Interventions
Within 28 days after screening, enrolled subjects underwent baseline evaluations, which included calculation of their DAS28-ESR. At the baseline visit, all subjects received their first dose of open-label RCI (1 ml, 80 U) and were observed for 1 h afterward. Subsequent open-label RCI doses were administered twice weekly at home by the subject or caregiver. Subjects returned to the study center at weeks 4, 8, and 12 (part 1) for efficacy and safety assessments. At week 12, subjects who had achieved LDA (DAS28-ESR < 3.2) were randomly assigned (in a 1:1 ratio) to receive either 1 ml (80 U) of RCI or 1 ml of placebo subcutaneously twice weekly during the 12-week double-blind period (part 2), which was designed to evaluate maintenance of response to therapy. Subjects who did not achieve LDA at week 12 were discontinued from further study participation.
During the double-blind period, subjects returned to the study center at weeks 16 and 24 for efficacy and safety assessments. Blood and urine samples for analysis of bone turnover markers were collected at weeks 12 and 24. At the follow-up visit, 28 days after the final dose of study drug, safety assessments were completed.
Bone turnover markers were analyzed by Eurofins Central Laboratory (Breda, The Netherlands). C-terminal crosslinking telopeptide (CTX), CTX-I, and N-terminal propeptide of type I collagen (PINP) were evaluated via electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN, USA); enzyme-linked immunosorbent assay was used to assess CTX-II (Immunodiagnostic Systems, East Boldon, UK), osteoprotegerin (OPG; Quidel, San Diego, CA, USA), and soluble receptor activator of NF-κB ligand (sRANKL; BioVendor, Karasek, Czech Republic). Creatinine was evaluated via Jaffe reaction using alkaline picrate (Roche Diagnostics, Indianapolis, IN, USA), and creatinine-adjusted CTX-II (CTX-II CRT) was calculated using the following formula:
Randomization and Blinding
Almac Clinical Technologies generated the randomization sequence, which used a block size of 4 and a 1:1 treatment allocation ratio. Four hundred randomization numbers (200 per treatment group) were generated, and randomization activities were conducted via the IXRS (interactive phone/web response system). A dummy subject randomization list was used for IXRS development and for Almac/client user acceptance testing. Except for those who prepared the randomization protocol and those involved in study drug preparation, all parties were blinded to subjects’ treatment conditions during the double-blind period.
Endpoints
The primary efficacy endpoint was the proportion of subjects who achieved DAS28-ESR < 3.2 at week 12 in part 1 of the study. Secondary efficacy endpoints included the proportion of subjects who maintained DAS28-ESR < 3.2 from week 12 through week 24; time to disease activity flare (as defined below) from weeks 12 through 24; the proportion of subjects with Clinical Disease Activity Index (CDAI) LDA (i.e., CDAI ≤ 10) [25] at weeks 12 and 24; and the proportion of subjects who met ACR criteria for 20% improvement (ACR20) at weeks 12 and 24. For weeks 13 through 23, disease activity flare was defined as meeting one of the following criteria: (1) DAS28-ESR < 3.2 and an increase of 1.2 from week 12; (2) DAS28-ESR ≥ 3.2 and an increase of > 0.6 from the week 12 assessment sustained over two consecutive visits; or (3) DAS28-ESR ≥ 3.2 and an increase of > 1 from the week 12 assessment at a single visit. Criteria 1 and 2 were based on validated criteria for RA flares [26, 27]; the third criterion was developed on the basis of the first two criteria, with slight modification for more stringency to capture potential flares not meeting criterion 1 or 2.
Exploratory endpoints included the proportions of subjects who achieved ACR50 and ACR70 responses at weeks 12 and 24 (with week 24 evaluated post hoc); the proportion of subjects with DAS28-ESR < 2.6 at weeks 12 and 24; and changes from baseline to weeks 12 and 24 in scores on the Health Assessment Questionnaire-Disability Index (HAQ-DI) [28], Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) [29], and Work Productivity and Activity Impairment (WPAI) scale [30]. Changes from baseline to weeks 12 and 24 in key markers of bone turnover (i.e., CTX, CTX-I, CTX-II, CTX-II CRT, PINP, OPG, sRANKL) were also evaluated as exploratory endpoints. Safety endpoints included AEs, vital signs, and laboratory test results, evaluated by study period and over the entire study.
Statistical Analysis
Screening of 360 subjects and enrollment of 232 were expected. An estimated 45% of enrolled subjects were expected to achieve DAS28-ESR < 3.2 at week 12 and continue on to be randomly assigned in part 2 of the study. On the basis of results from previous studies [31, 32], 80% of the RCI group and 50% of the placebo group were predicted to maintain LDA through week 24 (part 2). A sample size of 52 subjects per treatment group during part 2 was determined to provide 90% power to detect a difference between treatment groups using a two-sided, two-sample comparison of proportions at the significance level of 0.05.
The safety population included all enrolled subjects who received ≥ 1 dose of study drug. All subjects from the safety population who contributed any efficacy data to the study comprised the modified intent-to-treat (mITT) population, which was used for all efficacy analyses. All safety analyses were conducted using the safety population.
Data for all study variables were summarized via descriptive statistics. For the primary endpoint, the proportion of subjects with DAS28-ESR < 3.2 at week 12 (part 1), along with a two-sided 95% confidence interval (CI), was calculated. The study was deemed successful if the lower bound of the 95% CI was ≥ 10%.
The proportions of subjects with LDA defined by CDAI scores ≤ 10 at week 12 and the proportions of subjects who met ACR20, ACR50, or ACR70 criteria at week 12 were analyzed using the same method as the primary endpoint. Changes from baseline to week 12 for the DAS28-ESR, HAQ-DI, FACIT-F, WPAI, tender joint count, swollen joint count, and bone turnover markers were evaluated with one-sample t tests.
The proportions of subjects who maintained DAS28-ESR < 3.2 during part 2 (withdrawal phase, weeks 12–24) were compared across treatment groups using a Pearson’s Chi-square test, and the proportions of subjects who maintained DAS28-ESR < 2.6 and CDAI scores ≤ 10 and who met ACR20, ACR50, or ACR70 criteria were evaluated similarly. The time to disease activity flare in part 2 was analyzed using a log-rank test. Changes from baseline to week 24 for the DAS28-ESR, HAQ-DI, FACIT-F, WPAI, tender joint count, swollen joint count, and bone turnover markers were assessed via two-sample t tests.
Results
Participants
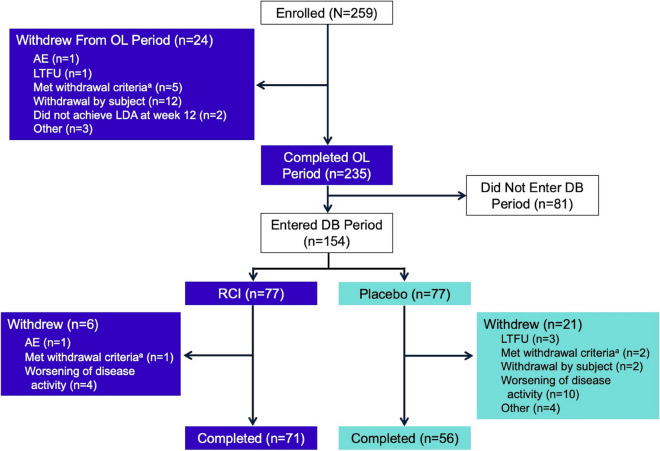
Of 259 enrolled subjects, 235 completed the open-label period and 127 completed the randomized, placebo-controlled, double-blind withdrawal period (Fig. 2). Subject demographics and baseline characteristics are shown in Table 1.
Fig. 2.
Subject disposition. aSubjects met withdrawal criteria if they developed a condition that met any of the study exclusion criteria or failed to meet any inclusion criteria during the study that was not considered an AE or if they were noncompliant. AE adverse event, DB double-blind, LDA low disease activity, LTFU lost to follow-up, OL open-label, RCI repository corticotropin injection
Table 1.
Subject demographics and baseline characteristics, safety population
| Characteristic | Part 1: open-label | Part 2: double-blind | |
|---|---|---|---|
| RCI (n = 259) | Placebo (n = 77) | RCI (n = 77) | |
| Age, mean (SD), years | 51.0 (12.2) | 50.9 (11.3) | 50.1 (12.2) |
| Female sex, no. (%) | 231 (89.2) | 69 (89.6) | 67 (87.0) |
| Race, no. (%) | |||
| White | 170 (65.6) | 53 (68.8) | 53 (68.8) |
| Black or African American | 15 (5.8) | 2 (2.6) | 1 (1.3) |
| Asian | 3 (1.2) | 1 (1.3) | 0 |
| Americana Indian or Alaska native | 40 (15.4) | 14 (18.2) | 12 (15.6) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 |
| Other | 31 (12.0) | 7 (9.1) | 11 (14.3) |
| Ethnicity, no. (%) | |||
| Hispanic or Latino | 213 (82.2) | 69 (89.6) | 73 (94.8) |
| Country, no. (%) | |||
| United States | 88 (34.0) | 19 (24.7) | 19 (24.7) |
| Mexico | 120 (46.3) | 46 (59.7) | 41 (53.2) |
| Argentina | 24 (9.3) | 7 (9.1) | 9 (11.7) |
| Peru | 27 (10.4) | 5 (6.5) | 8 (10.4) |
| Weight, mean (SD), kg | 72.9 (17.0) | 72.4 (14.5) | 70.8 (15.7) |
| BMI, mean (SD), kg/m2 | 28.8 (5.7) | 29.0 (5.4) | 28.2 (5.7) |
| Disease duration, mean (SD), years | 10.3 (8.0) | 9.4 (8.8) | 10.1 (6.8) |
| Prednisone (or equivalent) dose, mean (SD), mg/day | 6.3 (5.0) | 6.9 (8.7) | 5.9 (1.7) |
| Medical history of note, no. (%) [no. ongoing] | |||
| Hypertension | 74 (28.6) [73] | 20 (26.0) [20] | 20 (26.0) [20] |
| Obesity | 6 (2.3) [6] | 1 (1.3) [1] | 0 |
| Myocardial infarction | 2 (0.8) [0] | 1 (1.3) [0] | 0 |
| Arrhythmia | 1 (0.4) [1] | 0 | 0 |
| Cerebrovascular accident | 1 (0.4) [0] | 0 | 1 (1.3) [0] |
| Cerebrovascular disorder | 1 (0.4) [0] | 1 (1.3) [0] | 0 |
| Coronary artery disease | 1 (0.4) [1] | 0 | 0 |
| Type 2 diabetes mellitus | 1 (0.4) [1] | 1 (1.3) [1] | 0 |
| Methotrexate use, no. (%) | |||
| Prior | 253 (97.7) | 77 (100.0) | 77 (100.0) |
| Concomitant | 248 (95.8) | 77 (100.0) | 77 (100.0) |
| Most common (≥ 3% of subjects) prior DMARDs, no. (%) | |||
| Biologicb | 60 (23.2) | 7 (9.1) | 13 (16.9) |
| Adalimumab | 26 (10.0) | 3 (3.9) | 4 (5.2) |
| Etanercept | 22 (8.5) | 1 (1.3) | 4 (5.2) |
| Abatacept | 16 (6.2) | 1 (1.3) | 6 (7.8) |
| Certolizumab pegol | 13 (5.0) | 1 (1.3) | 2 (2.6) |
| Tocilizumab | 10 (3.9) | 0 | 2 (2.6) |
| Infliximab | 9 (3.5) | 1 (1.3) | 2 (2.6) |
| Nonbiologicc | 232 (89.6) | 74 (96.1) | 71 (92.2) |
| Hydroxychloroquine | 105 (40.5) | 26 (33.8) | 39 (50.7) |
| Sulfasalazine | 56 (21.6) | 19 (24.7) | 10 (13.0) |
| Leflunomide | 53 (20.5) | 20 (26.0) | 12 (15.6) |
| Chloroquine | 33 (12.7) | 13 (16.9) | 13 (16.9) |
| Tofacitinib | 8 (3.1) | 1 (1.3) | 3 (3.9) |
| Most common (≥ 3% of subjects) concomitant DMARDs, no. (%) | |||
| Biologicd | 45 (17.4) | 9 (11.7) | 17 (22.1) |
| Adalimumab | 12 (4.6) | 1 (1.3) | 1 (1.3) |
| Certolizumab pegol | 9 (3.5) | 1 (1.3) | 2 (2.6) |
| Etanercept | 9 (3.5) | 1 (1.3) | 1 (1.3) |
| Abatacept | 8 (3.1) | 1 (1.3) | 3 (3.9) |
| Nonbiologice | 224 (86.5) | 57 (74.0) | 59 (76.6) |
| Hydroxychloroquine | 97 (37.5) | 25 (32.5) | 38 (49.4) |
| Sulfasalazine | 54 (20.9) | 19 (24.7) | 9 (11.7) |
| Leflunomide | 46 (17.8) | 0 | 0 |
| Chloroquine | 33 (12.7) | 13 (16.9) | 13 (16.9) |
| DAS28-ESR, mean (SD) | 6.3 (1.0) | 6.2 (1.0) | 6.2 (0.9) |
| ESR, mean (SD) | 43.6 (24.8) | 42.0 (22.9) | 40.3 (21.5) |
| DAS28-ESR at week 12, mean (SD) | 3.6 (1.4) | 2.7 (0.5) | 2.8 (0.4) |
| ESR at week 12, mean (SD) | 24.0 (21.5) | 15.2 (12.6) | 15.8 (12.2) |
| Tender joint count, mean (SD)f | 14.7 (7.1) | 13.5 (7.2) | 13.5 (6.1) |
| Swollen joint count, mean (SD)f | 10.9 (5.4) | 10.1 (4.9) | 9.7 (4.3) |
| HAQ-DIf | 1.7 (0.6) | 1.7 (0.6) | 1.7 (0.5) |
| FACIT-Ff | 22.8 (8.4) | 22.6 (9.0) | 22.7 (7.7) |
BMI body mass index, DAS28 Disease Activity Score with 28 joint count, DMARD disease-modifying antirheumatic drug, ESR erythrocyte sedimentation rate, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, mITT modified intent-to-treat, RA rheumatoid arthritis, RCI repository corticotropin injection, SD standard deviation
aNorth, Central, or South American Indian
bGolimumab, rituximab, clazakizumab, sarilumab, and sirukumab each were taken by < 3% of subjects
cFilgotinib was taken by < 3% of subjects
dGolimumab, infliximab, and certolizumab each were taken by < 3% of subjects
eTofacitinib was taken by < 3% of subjects
fData are from the mITT population
Efficacy: Open-Label Period
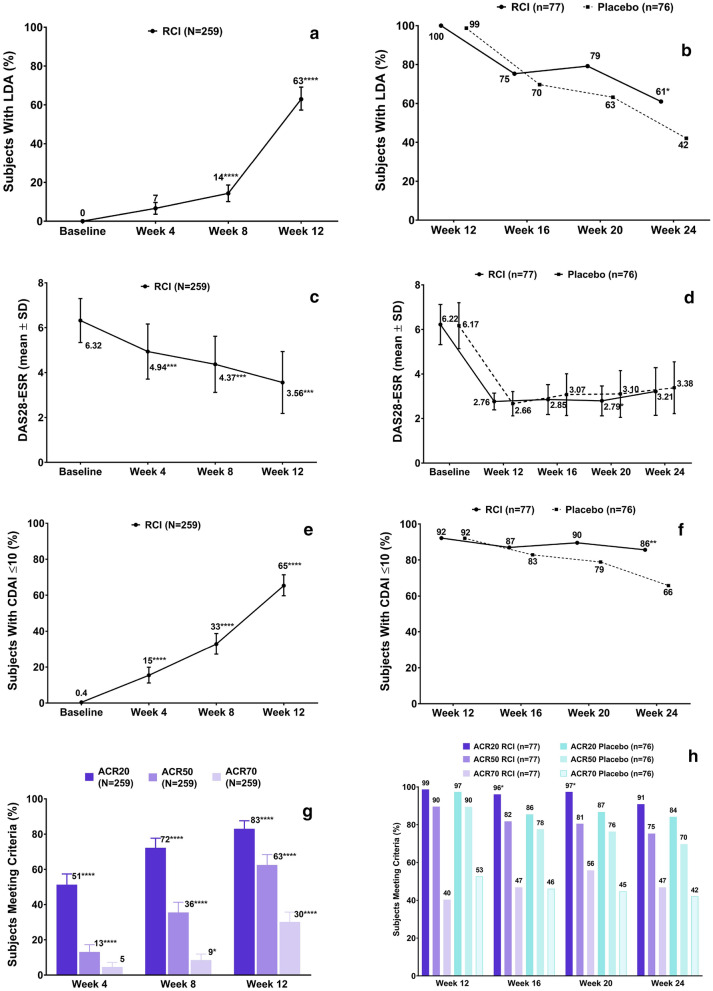
At week 12, 163 subjects [62.9% (95% CI 57.3–69.1%)] achieved DAS28-ESR < 3.2, the study’s primary endpoint (p < 0.0001; Fig. 3a). Figure 3c shows mean DAS28-ESR over time during the open-label period; mean change from baseline to week 12 was − 2.75 [standard deviation (SD), 1.45; p < 0.001]. Also at week 12, 169 subjects (65.3%) reached LDA, as defined by a CDAI score ≤ 10 (Fig. 3e), and 83.0% of subjects achieved ACR20, 62.5% achieved ACR50, and 30.1% achieved ACR70 (all p < 0.0001; Fig. 3g). Forty-nine subjects (18.9%) achieved DAS28-ESR < 2.6 (i.e., remission) at week 12. Levels of C-reactive protein did not change substantially (Figure S1a in the electronic supplementary material). Significant decreases from baseline in the number of tender and swollen joints were observed (Figure S2a in the electronic supplementary material).
Fig. 3.
Key efficacy outcomes (mITT population). Proportion of subjects achieving (part 1, open-label period) and maintaining (part 2, double-blind period) key efficacy milestones: LDA (DAS28-ESR < 3.2) (a, b), CDAI ≤ 10 (e, f), and ACR criteria (g, h). Mean DAS28-ESR over time (c, d). a Primary efficacy endpoint. *p ≤0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p < 0.0001 from one-sample binomial test (open-label period) or Pearson’s Chi-square test (double-blind period). p values denote differences from baseline for the open-label period and from placebo for the double-blind period. Percentages above bars are rounded to the nearest whole number. Error bars are 95% confidence intervals unless otherwise noted. Note: The proportions of subjects meeting ACR50 and ACR70 criteria during part 2 were not prespecified endpoints and were evaluated post hoc. ACR American College of Rheumatology, CDAI Clinical Disease Activity Index, DAS28-ESR Disease Activity Score with 28 joint count erythrocyte sedimentation rate, LDA low disease activity, mITT modified intent-to-treat, RCI repository corticotropin injection, SD standard deviation
Patient-Reported Outcomes: Open-Label Period
During part 1, open-label RCI therapy led to significant improvements from baseline in HAQ-DI (Figure S3a in the electronic supplementary material) and FACIT-F (Figure S4a in the electronic supplementary material) scores, as well as significant decreases from baseline in the percentages of work time missed, impairment while working, overall work impairment, and activity impairment, as assessed via the WPAI (Figure S5a in the electronic supplementary material).
Bone Turnover Markers: Open-Label Period
Most bone turnover markers were stable during the open-label period (Table 2), suggesting a minimal further impact of RCI on bone metabolism in patients already receiving glucocorticoids. At week 12, significant decreases in levels of cartilage degeneration markers CTX-II (p < 0.01) and CTX-II CRT (p < 0.001) as well as the bone formation marker PINP (p < 0.01) were observed, and bone degeneration markers CTX and CTX-I showed no significant changes with RCI treatment in this population.
Table 2.
Bone turnover markers, mITT population
| Time point | Marker, mean (SD) | ||||||
|---|---|---|---|---|---|---|---|
| CTX, μg/l |
CTX-I, μg/l |
CTX-II, μg/l |
CTX-II CRT, ng/mmol |
OPG, pmol/l |
PINP, μg/l |
sRANKL, pmol/l |
|
| Open-label period | |||||||
| Baselinea | 4.79 (2.09) | 0.39 (0.21) | 3.46 (2.31) | 452.4 (325.4) | 4.71 (1.80) | 52.23 (28.21) | 2057.70 (3592.90) |
| Week 12b | 4.76 (1.93) | 0.39 (0.21) | 2.99c (2.17) | 362.5d (273.1) | 4.68 (1.98) | 47.37c (26.21) | 2107.55 (3794.56) |
| Double-blind period | |||||||
| Baseline | |||||||
| RCIe | 4.77 (1.89) | 0.44 (0.22) | 3.69 (2.47) | 463.7 (316.9) | 4.86 (1.83) | 54.76 (28.79) | 1519.42 (2378.26) |
| Placebof | 4.58 (1.98) | 0.38 (0.18) | 3.61 (2.42) | 460.5 (368.3) | 4.65 (1.78) | 52.46 (26.38) | 2416.34 (3825.88) |
| Week 12 | |||||||
| RCIg | 4.58 (1.40) | 0.45 (0.23) | 2.93 (2.19) | 368.0 (228.6) | 4.79 (2.23) | 51.19 (29.06) | 2451.77h (4417.55) |
| Placeboi | 4.61 (1.63) | 0.40 (0.21) | 3.21 (2.36) | 382.5 (257.5) | 4.73 (1.89) | 48.69 (25.07) | 2358.63 (4401.72) |
| Week 24 | |||||||
| RCIj | 4.79 (2.76) | 0.44 (0.20) | 3.13 (1.87) | 339.4 (189.7) | 4.93 (2.04) | 54.34 (40.08) | 2938.96k (5006.25) |
| Placebol | 4.47 (1.68) | 0.41 (0.20) | 3.27 (2.05) | 391.6 (236.0) | 5.12 (2.12) | 53.10 (26.16) | 2105.64 (4116.93) |
Bolded values are statistically significant
CTX C-terminal crosslinking telopeptide, CTX-I C-terminal crosslinking telopeptide of type I collagen, CTX-II C-terminal crosslinking telopeptide of type II collagen, CTX-II CRT creatinine-adjusted CTX-II, mITT modified intent-to-treat, OPG osteoprotegerin, PINP N-terminal peptide of type I collagen, RCI repository corticotropin injection, SD standard deviation, sRANKL soluble receptor activator of nuclear kappa B ligand
aCTX, n = 251; CTX-I and OPG, n = 254; CTX-II, n = 190; CTX-II CRT, n = 183; PINP, n = 257; sRANKL, n = 250
bCTX, n = 238; CTX-I, n = 243; CTX-II, n = 159; CTX-II CRT, n = 153; OPG, n = 239; PINP, n = 246; sRANKL, n = 231
cp < 0.01, one-sample t test for week 12 versus baseline
dp < 0.001, one-sample t test for week 12 versus baseline
eCTX, CTX-I, and PINP, n = 75; CTX-II, n = 59; CTX-II CRT, n = 57; OPG and sRANKL, n = 73
fCTX, n = 72; CTX-I, OPG, and sRANKL, n = 75; CTX-II, n = 59; CTX-II CRT, n = 57; PINP, n = 76
gCTX, CTX-I, and PINP, n = 75; CTX-II, n = 66; CTX-II CRT, n = 64; OPG, n = 74; sRANKL, n = 72
hp < 0.05, two-sample t test for RCI time point versus baseline
iCTX, n = 73; CTX-I, OPG, and PINP, n = 74; CTX-II, n = 62; CTX-II CRT, n = 61; sRANKL, n = 71
jCTX, CTX-I, and PINP, n = 75; CTX-II and CTX-II CRT, n = 63; OPG, n = 74; sRANKL, n = 70
kp < 0.01, two-sample t test for RCI time point versus baseline
lCTX, CTX-I, OPG, and PINP, n = 65; CTX-II, n = 46; CTX-II CRT, n = 45; sRANKL, n = 61
Efficacy: Double-Blind Withdrawal Period
At week 24, DAS28-ESR LDA was maintained in 47 of 77 (61.0%) RCI-treated subjects and 32 of 76 (42.1%) placebo-treated subjects (p = 0.019; Fig. 3b). Mean DAS28-ESR over time during the double-blind period did not differ significantly between treatment groups (Fig. 3d). Mean time to disease activity flare during weeks 12 through 24 was 6.5 weeks (SD, 2.61 weeks) for the placebo group and 8.2 weeks (SD, 2.92 weeks) for the RCI group. At week 24, 66 subjects (85.7%) in the RCI group and 50 (65.8%) in the placebo group maintained LDA, as defined by CDAI scores ≤ 10 (p = 0.004; Fig. 3f). A vast majority of subjects achieved ACR20 and ACR50 responses during the open-label period, and these responses were maintained through the double-blind period in both treatment groups; at week 24 of the double-blind period, ACR70 responses were seen in 47% of RCI-treated subjects and 42% of subjects who had discontinued RCI (Fig. 3h). At week 24, 23 subjects (29.9%) in the RCI group and 23 (30.3%) in the placebo group achieved DAS28-ESR remission (p = 0.828) in this population with previously highly active RA. Levels of C-reactive protein did not change substantially (Figure S1b in the electronic supplementary material). The mean number of tender and swollen joints remained decreased during the double-blind period, with no significant differences between the RCI and placebo groups noted (Figure S2b-c in the electronic supplementary material).
Patient-Reported Outcomes: Double-Blind Period
Improvements on the HAQ-DI, FACIT-F, and WPAI that were noted during the open-label period were generally maintained in both treatment groups throughout the double-blind period (Figures S3b, S4b, and S5b–e in the electronic supplementary material). There were no significant differences between the RCI and placebo groups on these metrics.
Bone Turnover Markers: Double-Blind Period
Levels of the osteoclast differentiation marker sRANKL significantly increased from baseline to week 12 and week 24 (both p < 0.05) in the RCI group, but not in the placebo group (Table 2). All other bone turnover markers remained stable.
Safety
During the open-label period, 43 subjects (16.6%) reported treatment-related AEs; nine subjects (11.7%) in the RCI group and ten (13.0%) in the placebo group reported treatment-related AEs during the double-blind period (Table 3). Adverse events that are typically associated with corticosteroid use (e.g., hypertension, hyperglycemia, headache, weight gain, edema) were low in incidence or not reported at all. Three subjects (1.2%) reported serious AEs, all during the open-label period. One case each of chest pain and craniocerebral injury were considered unrelated to treatment. One case of pneumonia was considered possibly related to treatment; RCI therapy was discontinued, and the patient recovered. No subjects died during the study.
Table 3.
Summary of AEs, safety population
| Part 1 (open-label period) | |
|---|---|
| AE | No. (%) of patients RCI (n = 259) |
| Any AEa | 98 (37.8) |
| Anemia | 5 (1.9) |
| Glycosylated hemoglobin increased | 4 (1.5) |
| Headache | 9 (3.5) |
| Hypertension | 4 (1.5) |
| Nasopharyngitis | 4 (1.5) |
| Nausea | 5 (1.9) |
| Pharyngitis | 7 (2.7) |
| Upper respiratory tract infection | 4 (1.5) |
| Urinary tract infection | 10 (3.9) |
| AE resulting in study drug withdrawal | 3 (1.2) |
| Serious AE | 3 (1.2) |
| Serious infectious event | 1 (0.4) |
| Opportunistic infections | |
| Herpes zoster | 1 (0.4) |
| Tuberculosis | 0 |
| Death | 0 |
| Part 2 (double-blind period) | ||
|---|---|---|
| AE | No. (%) of patients | |
| Placebo (n = 77) | RCI (n = 77) | |
| Any AEa | 31 (40.3) | 25 (32.5) |
| Anemia | 2 (2.6) | 2 (2.6) |
| Back pain | 0 | 2 (2.6) |
| Diarrhea | 3 (3.9) | 1 (1.3) |
| Dizziness | 1 (1.3) | 1 (1.3) |
| Gastritis | 2 (2.6) | 1 (1.3) |
| Glycosylated hemoglobin increasedb | 2 (2.6) | 1 (1.3) |
| Headache | 5 (6.5) | 5 (6.5) |
| Hyperglycemia | 2 (2.6) | 3 (3.9) |
| Hypertension | 0 | 3 (3.9) |
| Influenza | 1 (1.3) | 1 (1.3) |
| Nasopharyngitis | 2 (2.6) | 2 (2.6) |
| Rhinitis | 2 (2.6) | 0 |
| Upper respiratory tract infection | 3 (3.9) | 0 |
| Urinary tract infection | 3 (3.9) | 2 (2.6) |
| AE resulting in study drug withdrawal | 1 (1.3) | 0 |
| Serious AE | 0 | 0 |
| Serious infectious event | 0 | 0 |
| Opportunistic infections | ||
| Herpes zoster | 0 | 0 |
| Tuberculosis | 0 | 0 |
| Death | 0 | 0 |
AE adverse event, RCI repository corticotropin injection
aAEs reported in ≥ 1.5% of subjects in part 1 or in either group in part 2 are listed below
bRefers to glycosylated hemoglobin values > 6.5%
Discussion
These results support the efficacy of RCI in patients with continued highly active RA despite treatment with prednisone and one or two DMARDs, which could include a bDMARD. Despite a mean baseline DAS28-ESR of 6.3, 63% of patients achieved DAS28-ESR < 3.2 by week 12, with a statistically significant percentage achieving LDA by week 8. Thus, the study’s primary endpoint was met (Fig. 3a). These results were confirmed by the proportions of subjects who achieved CDAI ≤ 10 (Fig. 3e) and ACR20, ACR50, and ACR70 responses (Fig. 3g) during the open-label period.
During the double-blind period, DAS28-ESR LDA was maintained in almost two-thirds of subjects who continued RCI. Of importance, > 40% of patients who withdrew from RCI to placebo maintained LDA for an additional 12 weeks (Fig. 3b). These results suggest that patients with high disease activity despite treatment with glucocorticoids and a csDMARD (with or without a bDMARD) or a bDMARD as monotherapy may have a clinically meaningful decrease in disease activity with RCI treatment for a period of 3 months, which may be maintained even after RCI treatment withdrawal. Interestingly, when LDA was assessed by CDAI ≤ 10 (Fig. 3d), 86% of subjects who continued on RCI maintained LDA, and almost two-thirds of subjects who withdrew RCI maintained LDA. Disparity in the durability of response as assessed by DAS28-ESR versus by CDAI is most likely explained by differences in the contribution of various elements to these metrics. Although both the DAS28 and the CDAI assess tender and swollen joints, these factors are given equal weight in calculation of CDAI scores, whereas tender joint counts are given twice the weight of swollen joints in calculation of the DAS28-ESR [25, 33]. Importantly, the CDAI does not assess ESR, an acute phase reactant, whereas the DAS28-ESR does.
Achievement and maintenance of remission, or at least LDA, should be the primary approach for RA, but assessment of physical functioning, disability, and other health outcomes also provides important information for the overall evaluation of a drug’s benefit. During the open-label period, improvements were seen in several patient-reported outcomes assessing disability (HAQ-DI), fatigue (FACIT-F), and work/activity (WPAI). These improvements were generally maintained during the double-blind withdrawal period in both treatment groups, which further supports the suggestion that the benefits of RCI may be maintained for some time after treatment is discontinued.
No unexpected safety signals were observed during the study. The three serious AEs reported in patients receiving RCI were consistent with those previously reported with RCI therapy. The incidences of AEs commonly associated with corticosteroids were low and typically similar in the RCI and placebo groups, which suggests the possibility of minimal additional steroidal effects of RCI in patients already on glucocorticoids. One might expect a greater incidence of common corticosteroid-associated AEs if RCI therapy were continued indefinitely, but extension studies and/or registries would be needed to evaluate the safety of long-term RCI therapy. Markers of bone turnover were mostly stable during both study periods, indicating no pronounced additional effect of RCI on bone metabolism in patients who had been receiving 5 to 10 mg/day of prednisone (or equivalent) for ≥ 4 weeks. In the open-label period, cartilage degeneration markers showed a significant decrease from baseline at week 12; although the bone formation marker PINP significantly decreased, this may not be indicative of bone loss, especially because bone degeneration markers remained stable. During the double-blind period, levels of osteoclast marker sRANKL significantly increased from baseline to weeks 12 and 24 in the RCI group but not the placebo group. However, this may not suggest evidence of bone damage, as bone degeneration marker levels remained stable. The role of RCI and bone turnover in a population already receiving glucocorticoids over a prolonged period still needs to be evaluated.
Results from this study suggest a role for RCI in treating patients who have active RA with at least moderate disease activity despite maximal treatment with DMARDs, whether csDMARDs or bDMARDs, and who are also being treated with glucocorticoids. In previous studies, including the COBRA study [34] and other similar trials, high- or moderate-dose glucocorticoids were used in the initiation of therapy (with subsequent tapering) to quickly obtain disease control. In contrast, RCI was used not as initial therapy but rather as rescue therapy in the current study while low/moderate-dose glucocorticoids were maintained. Assuming the patient has been maximally treated with DMARDs, a potential treatment scenario might involve 3 months of RCI therapy with the aim of achieving remission or LDA. Results from this study suggest that such patients with recalcitrant disease may respond well to RCI therapy and that some patients will maintain LDA after discontinuation of RCI therapy. Thus, it is at the discretion of the physician whether to discontinue RCI therapy at 3 months, with subsequent monitoring to assess whether response is maintained. For patients who develop a flare after RCI discontinuation, the consideration of additional RCI therapy for flares when they occur may be reasonable if long-term safety findings support such an approach. The effects of long-term RCI therapy on bone health and other aspects of safety are unclear and require further study, but the results from this study suggest that RCI could be used in an intermittent manner for many patients without significant concerns about general safety or bone loss. For patients who have flares shortly after discontinuation of RCI therapy, the risk–benefit for prolonged use of RCI still needs to be defined.
This study has some notable limitations. Although we used a study design that has previously been employed successfully [35], the primary endpoint was measured in the open-label period, during which all subjects knew that they were being treated with RCI. The response observed for this endpoint in this population with recalcitrant disease was higher than expected, which could, in part, be a result of the study design (i.e., a placebo effect). However, it would be unusual for a placebo effect to manifest after 8 weeks, as it was in this study (Fig. 3a, e). In addition, the vast majority (> 80%) of study participants were of Hispanic or Latino ethnicity, which may limit extrapolation of the results to the general population. It is also worth noting that patients with other rheumatic autoimmune diseases, clinically significant infections, or malignancies were excluded from the study. Caution should be observed in extrapolating the study’s safety results to such populations. Also, bone density testing was not performed in this study but would be reasonable to evaluate in future studies, considering the association between glucocorticoid therapy and the development of osteoporosis. Finally, participants in the current study had highly active RA despite treatment with DMARDs and glucocorticoids, and changes in these baseline therapies were not allowed during the study in order to properly assess the rescue effects of RCI. Current guidelines from EULAR [36] and ACR [4] recommend that glucocorticoids be used at the lowest possible dose and tapered as soon as feasible. Future studies with additional treatment arms wherein tapering of glucocorticoids and/or DMARD adjustments are allowed may be warranted.
Despite these limitations, the randomized, double-blind, placebo-controlled withdrawal period of this study provided a more rigorous assessment of the efficacy and safety of RCI.
Conclusions
The results of this study of patients who had active RA despite corticosteroid/DMARD therapy show that patients can achieve LDA as early as week 8. By week 12, more than half of the subjects achieved LDA, which was maintained with an additional 12 weeks of treatment. Importantly, many subjects who achieved LDA with RCI therapy and then discontinued RCI use during the withdrawal period were able to maintain LDA for the subsequent 3 months, which may suggest sustained durability of RCI therapy. No new unexpected safety signals were noted, and markers of bone turnover were mostly stable, suggesting that RCI does not cause further bone loss in patients with active RA previously treated with glucocorticoids.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
This work, including the journal’s Rapid Service and Open Access fees, was supported by Mallinckrodt Pharmaceuticals.
Medical Writing and Editorial Assistance
Technical and editorial support for this manuscript was provided by Elizabeth Barton, MS, of MedLogix Communications, LLC, and funded by Mallinckrodt Pharmaceuticals.
Authorship
All named authors meet International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
This manuscript is based on work previously presented at the European Congress of Rheumatology in Madrid, Spain, June 12-15, 2019.
Disclosures
Roy Fleischmann has received clinical trial grants from AbbVie, Acea, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Genentech, Gilead, GSK, Janssen, Eli Lilly, Merck, Pfizer, Roche, Sanofi, Aventis, Samumed, and UCB. He has received consultancy fees from AbbVie, Acea, Akros, Amgen, BMS, Celltrion, Galvani, Gilead, GSK, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Sandoz, Sanofi Aventis, Taiho, and UCB, as well as advisory fees (not related to this study) from Mallinckrodt Pharmaceuticals. Roy Fleischmann is also an Editor-in-Chief of the journal. Daniel E. Furst has received grants and consultancy fees from AbbVie, Actelion, Amgen, BMS, Corbus Pharmaceuticals, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. He has also received consultancy fees from Cytori Therapeutics and speaking fees from CMC Connect (McCann Health Company) and is a member of the journal’s Editorial Board. Erin Connolly-Strong, Jingyu Liu and Julie Zhu are employees of Mallinckrodt Pharmaceuticals. Richard Brasington has served as a speaker for Amgen, Mallinckrodt Pharmaceuticals, Novartis, and Pfizer.
Compliance with Ethics Guidelines
The study was conducted at 60 centers in four countries (see electronic supplementary material for details on study centers) from November 7, 2016, to February 13, 2019, in accordance with the principles and requirements of the Declaration of Helsinki, Good Clinical Practices, and clinical trial registration (clinicaltrials.gov identifier NCT02919761). All investigators obtained institutional review board/independent ethics committee approval. All subjects provided informed consent (including consent for publication) before any study procedures were performed.
Data Availability
The discussion of statistical endpoints and analysis is included in this manuscript. Summary aggregate results, including AE information and the study protocol, will be available by December 2019 on ClinicalTrials.gov (NCT02919761). Individual patient data will not be disclosed unless requested, allowed per informed consent, and appropriately anonymized. Requests for additional information should be directed to Mallinckrodt Pharmaceuticals’ Department for Clinical Trial Disclosure and Transparency at clinicaltrials@mnk.com.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to: 10.6084/m9.figshare.11903241.
References
- 1.Zerbini CAF, Clark P, Mendez-Sanchez L, Pereira RMR, Messina OD, Uña CR, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017;28(2):429–446. doi: 10.1007/s00198-016-3769-2. [DOI] [PubMed] [Google Scholar]
- 2.Akil M, Moots R. Rheumatoid arthritis: clinical features and diagnosis. In: Adebajo A, Dunkley L, editors. ABC of rheumatology. 5. Oxford: Wiley; 2018. pp. 73–76. [Google Scholar]
- 3.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- 7.Donahue KE, Gartlehner G, Schulman ER, Jonas B, Coker-Schwimmer E, Patel SV, et al. Drug therapy for early rheumatoid arthritis: a systematic review update. Comparative Effectiveness Review No. 211. (Prepared by the RTI International–University of North Carolina at Chapel Hill Evidence-Based Practice Center for AHRQ and PCORI). Rockville, MD: Agency for Healthcare Research and Quality July 2018. Contract No.: 290-2015-00011-I. AHRQ Publication No. 18-EHC015-EF. PCORI Publication No. 2018-SR-02.
- 8.Bijlsma JW, Boers M, Saag KG, Furst DE. Glucocorticoids in the treatment of early and late RA. Ann Rheum Dis. 2003;62(11):1033–1037. doi: 10.1136/ard.62.11.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acthar Gel [package insert]. Bedminster: Mallinckrodt Pharmaceuticals; 2019.
- 10.Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Sci World J. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed TJ, Montero-Melendez T, Perretti M, Pitzalis C. Curbing inflammation through endogenous pathways: focus on melanocortin peptides. Int J Inflamm. 2013;2013:985815. doi: 10.1155/2013/985815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott RJ, Szabo M, Wagner MJ, Kemp EH, MacNeil S, Haycock JW. alpha-Melanocyte-stimulating hormone, MSH 11-13 KPV and adrenocorticotropic hormone signalling in human keratinocyte cells. J Investig Dermatol. 2004;122(4):1010–1019. doi: 10.1111/j.0022-202X.2004.22404.x. [DOI] [PubMed] [Google Scholar]
- 13.Manna SK, Aggarwal BB. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol. 1998;161(6):2873–2880. doi: 10.4049/jimmunol.161.6.2873. [DOI] [PubMed] [Google Scholar]
- 14.Cooray SN, Clark AJ. Melanocortin receptors and their accessory proteins. Mol Cell Endocrinol. 2011;331(2):215–221. doi: 10.1016/j.mce.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Starowicz K, Przewlocka B. The role of melanocortins and their receptors in inflammatory processes, nerve regeneration and nociception. Life Sci. 2003;73(7):823–847. doi: 10.1016/S0024-3205(03)00349-7. [DOI] [PubMed] [Google Scholar]
- 16.Grässel S, Opolka A, Anders S, Straub RH, Grifka J, Luger TA, et al. The melanocortin system in articular chondrocytes: melanocortin receptors, pro-opiomelanocortin, precursor proteases, and a regulatory effect of alpha-melanocyte-stimulating hormone on proinflammatory cytokines and extracellular matrix components. Arthritis Rheum. 2009;60(10):3017–3027. doi: 10.1002/art.24846. [DOI] [PubMed] [Google Scholar]
- 17.Patel HB, Bombardieri M, Sampaio AL, D’Acquisto F, Gray M, Grieco P, et al. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J. 2010;24(12):4835–4843. doi: 10.1096/fj.10-167759. [DOI] [PubMed] [Google Scholar]
- 18.Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, et al. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36(5):820–831. doi: 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Fischer PA, Rapoport RJ. Repository corticotropin injection in patients with rheumatoid arthritis resistant to biologic therapies. Open Access Rheumatol. 2018;10:13–19. doi: 10.2147/OARRR.S153307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaylis N, Needell S, Sagliani J. The effect of corticotropin (ACTH 80 units weekly or biweekly) in combination with MTX in newly diagnosed RA patients from a clinical and structural perspective as measured by a CDAI score and osteitis, synovitis, and erosions on MRI. Ann Rheum Dis. 2015;74(Suppl 2):1066–1067. doi: 10.1136/annrheumdis-2015-eular.2810. [DOI] [Google Scholar]
- 21.Gillis T, Crane M, Hinkle C, Wei N. Repository corticotropin injection as adjunctive therapy in patients with rheumatoid arthritis who have failed previous therapies with at least three different modes of action. Open Access Rheumatol. 2017;9:131–138. doi: 10.2147/OARRR.S131046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann Rheum Dis. 2007;66(3):407–409. doi: 10.1136/ard.2006.054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 25.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–S108. [PubMed] [Google Scholar]
- 26.van der Maas A, Lie E, Christensen R, Choy E, de Man YA, van Riel P, et al. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis. 2013;72(11):1800–1805. doi: 10.1136/annrheumdis-2012-202281. [DOI] [PubMed] [Google Scholar]
- 27.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41(10):1845–1850. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 28.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32(5):811–819. [PubMed] [Google Scholar]
- 30.Zhang W, Bansback N, Boonen A, Young A, Singh A, Anis AH. Validity of the work productivity and activity impairment questionnaire-general health version in patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12(5):R177. doi: 10.1186/ar3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pincus T. The clinical efficacy of 3 mg/day prednisone in patients with rheumatoid arthritis: evidence from a randomized, double-blind, placebo-controlled withdrawal clinical trial. Clin Exp Rheumatol. 2011;29(5 Suppl 68):S73–S76. [PubMed] [Google Scholar]
- 32.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 33.DAS(28) in depth. http://www.das-score.nl/das28/en/difference-between-the-das-and-das28/das-28-in-depth.html?tmpl=component&print=1&page. Accessed Feb 7, 2019.
- 34.Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, van Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350(9074):309–318. doi: 10.1016/S0140-6736(97)01300-7. [DOI] [PubMed] [Google Scholar]
- 35.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342(11):763–769. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 36.Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The discussion of statistical endpoints and analysis is included in this manuscript. Summary aggregate results, including AE information and the study protocol, will be available by December 2019 on ClinicalTrials.gov (NCT02919761). Individual patient data will not be disclosed unless requested, allowed per informed consent, and appropriately anonymized. Requests for additional information should be directed to Mallinckrodt Pharmaceuticals’ Department for Clinical Trial Disclosure and Transparency at clinicaltrials@mnk.com.