Figure 6. Ablation of Pgr Causes Increased Oxidative Stress and Inflammatory Damage in the Ovary.
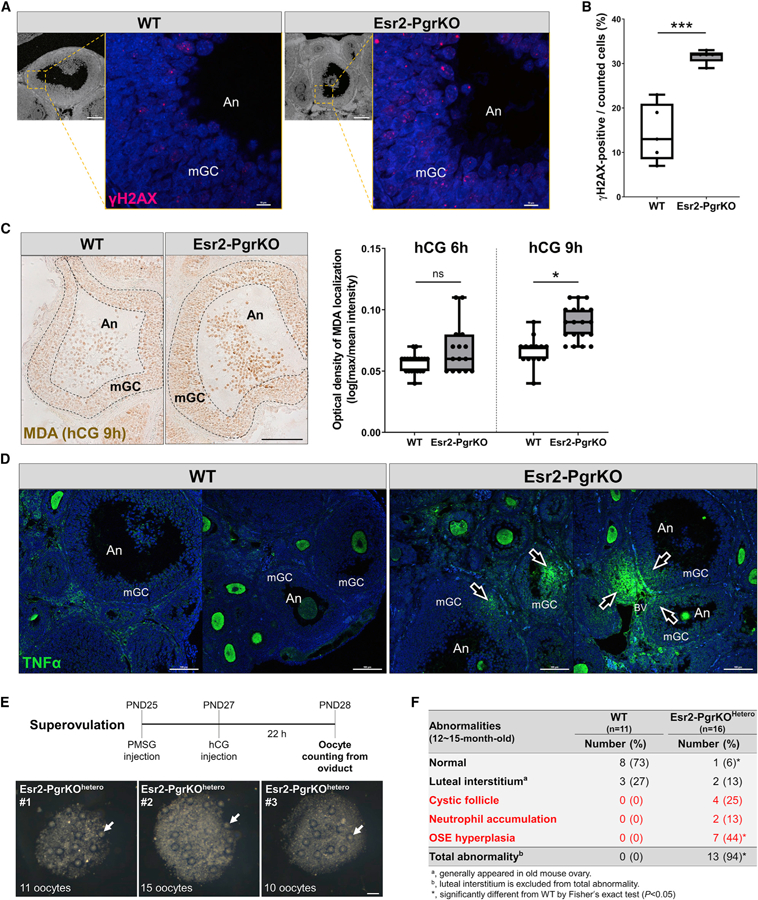
Mice were injected with hCG following PMSG injection (48 h). Ovaries were collected from WT and Esr2-PgrKO mice at 6 h after hCG injection and subjected to immunohistochemistry or immunofluorescent examination.
(A) Immune-localization of γH2AX (red) in antral follicles of WT and Esr2-PgrKO ovaries (scale bars in bottom magnification, 0.1 mm; scale bars in top magnification, 10 µm).
(B) Quantitative analysis of γH2AX-positive cells in mural granulosa cell layer of antral follicles. Five different antral follicles in WT and Esr2-PgrKO were subjected to quantitative analysis. Error bars, SD (n = 5); ***p < 0.001 (Student’s t test).
(C) Immune-localization of malondialdehyde (brown) in ovarian follicles of WT and Esr2-PgrKO. Optical intensity of the brown channel was quantified and compared between WT and Esr2-PgrKO at hCG 6 and 9 h (scale bar, 50 µm). Error bars, SD (n = 12–20); *p < 0.01 (Student’s t test).
(D) Immune-localization of TNFα (green) in WT and Esr2-PgrKO ovaries. Arrows indicate TNFα invasion into the follicles. An, Antrum; mGC, mural granulosa cells (scale bars, 100 µm).
(E) The number of ovulated oocytes was counted in Pgrflox/wtEsr2iCre/wt after inducing of superovulation. Arrows indicate the ovulated oocytes from a single ovary (scale bars, 100 µm).
(F) Frequency of occurrence of major abnormalities in WT and Esr2-PgrKOhetero ovaries at the age of 12–15 months after birth.
