Abstract
Objective
To evaluate the effect of strategies to reduce the spread of simulated aerosol during chest compressions on manikin and cadaver experimental models.
Methods
To evaluate aerosol-spread we nebulized ultraviolet sensitive detergents into the artificial airway of a resuscitation dummy and performed CPR. The spread of the visualized aerosol was documented by a camera. In a further approach we applied nebulized detergents into the airways of human cadavers and detected the simulated spread on the same way. Among others we did recordings with undergoing compression-only-CPR, with a surgical mask or an oxygen mask on the patients face and with an inserted supraglottic airway device with and without a connected airway filter.
Results
Most aerosol-spread at the direction of the provider was visualized during compression-only-CPR. The use of a surgical mask and of an oxygen mask on the patient's face deflected the spread. Inserting a supraglottic airway device connected to an airway filter lead to a remarkable reduction of aerosol-spread.
Conclusion
The early insertion of a supraglottic airway device connected to an airway filter before starting chest compression may be beneficial for staff protection during CPR.
Introduction
The Coronavirus Disease 2019 (COVID-19) spread all over the world and became pandemic.1 There is a risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for medical professionals during patient care, particularly in aerosol-generating procedures.1, 2, 3 It is uncertain, if chest compressions are an aerosol generating procedure. A recent review found neither evidence for nor evidence against aerosol production and infection risk during chest compressions.4 Dealing with uncertainty is part of dealing with a pandemic. According to data from China, where SARS-CoV-2 emerged, the spectrum of disease (N = 44,415) was severe in 14% (6168 cases) and critical in 5% (2087 cases).5 In Italy, 9% (2026 cases) of all COVID-19 cases reported in March (22,512 cases) were health care workers.6 Adequate protection especially during aerosol-generating procedures is of utmost importance. Currently there is little data regarding safety precautions during cardiopulmonary resuscitation. There are different recommendations for staff protection during CPR, e.g. using a face mask or an oxygen mask on the patients face.7 The purpose of this study was to visualize artificially generated aerosol-spread during chest compressions with different strategies of staff protection and airway management.
Methods
CPR simulation model
In a first approach, we created an ex situ simulation model using a modified resuscitation dummy. A difficult airway trainer was connected to two identical pediatric self-inflating bags (Ambu® SPUR® II, Ambu GmbH, Bad Nauheim, Germany) to simulate the lung placed in a chest compression trainer. We connected a nebulizer for inhalation (Cirrus™ 2, Intersurgical GmbH, Sank Augustin, Germany) at the connection port for the reservoir bag of the self-inflating bag (Fig. 1 panel A). To visualize aerosol-spread we nebulized different disinfection detergents detectable by ultraviolet light (Ecolab® magic blue, Ecolab Deutschland GmbH, Monheim am Rhein, Germany; Bode visirub® conc., Bode Chemie GmbH, Hamburg, Germany; Schuelke S&M® Optik, Schuelke & Mayr GmbH, Wien, Oesterreich). While applying an air flow of 8 l/min to the nebulizer, chest compression on the resuscitation dummy was performed. The airflow of 8 l/min was the minimum flow required to generate adequate aerosol for visualization using ultraviolet sensitive detergents with a nebulizer.
Fig. 1.
Details of the experimental setup of the simulation model and the cadaver model.
Cadaver model
In a second approach we visualized aerosol-spread during CPR in a human cadaver model in cooperation with the Institute of Clinical Anatomy and Cell Analysis, Eberhard Karls University Tuebingen. Ethical approval was given by the ethics committee of the University of Tuebingen (Project No.: 226/2020BO2). The human cadavers were fixed with an ethanol-based technique. To fill the airway and lungs with ultraviolet sensitive detergent, we used a commonly used self-inflating bag (Ambu® SPUR® II, Ambu GmbH, Bad Nauheim, Germany) connected to a nebulizer set (DAR™ Nebulizer Set, Covidien Ireland Limited, Tullamore, Ireland) and a shortened endotracheal tube with 7 mm internal diameter (Mallinckrodt™ Hi-Contour Oral/Nasal Tracheal Tube Cuffed, Covidien Ireland Limited, Tullamore, Ireland). We insufflated aerosol until it was visible at the labial angle of the cadaver. These devices have been removed from the airway before starting chest compression.
Data collection and study protocol
We documented the spread of the luminescent aerosol by taking photos in a dark room with ultraviolet lamps. The aerosol, simulated by nebulized ultraviolet sensitive detergents, spreading during ongoing chest compressions was photo documented in different scenarios. We used a Nikon Df digital camera with a Sigma DG HSM Nikon Art 24.0 mm f/1.4 lens. Camera settings including ISO and shutter speed have been set once for CPR simulation model and cadaver model. During chest compression, photos were taken with a frame rate of 5.5 frames per second from a fixed angle. In our study protocol we analyzed the potential risk by choosing the picture showing the most aerosol-spread for each setting. At first, aerosol-spread was detected during compression-only-CPR. In a second approach, we attached a face mask followed by an oxygen mask with reservoir on the patients face. Afterwards we inserted supraglottic airway devices, such as a laryngeal tube (LTS-D, VBM Medizintechnik GmbH, Sulz a. N., Germany) and different laryngeal masks (i-gel®, Intersurgical GmbH, Sank Augustin, Germany; Ambu® AuraOnce™, Ambu GmbH, Bad Nauheim, Germany). In a stepwise approach we tested all supraglottic airway devices connected and not connected to an airway filter (DAR™ Adult-Pediatric Electrostatic Filter HME, Covidien Ireland Limited, Tullamore, Ireland). Additionally, the aerosol-spread of intermittent bag-mask ventilation and during laryngoscopy was visualized. Since three cadavers were available for our study, we could only demonstrate limited techniques on the cadaver model due to the rapid deterioration of the mechanical properties of the lung during CPR. The other techniques were visualized while using the ex situ model. We evaluated the extend and direction of the aerosol-spread by visual estimation based on the acquired pictures. Fig. 1 shows details of the experimental setup.
Results
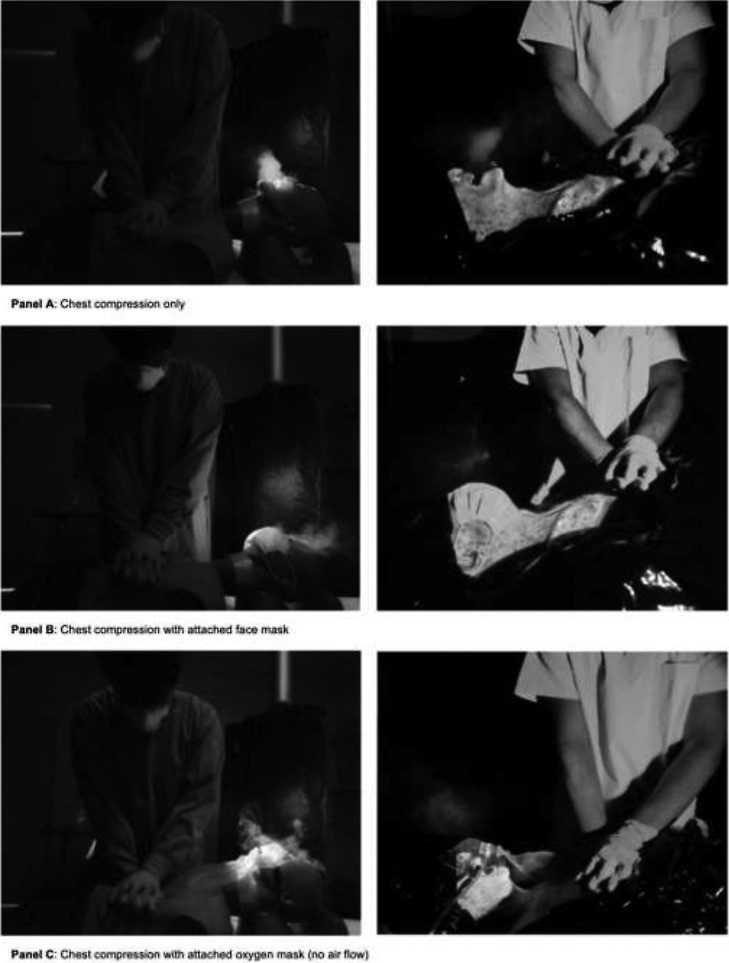
In our study most spread particularly into the direction of the provider of chest compression was recognized during compression-only-CPR without any airway device as shown in Fig. 2, Fig. 3 .
Fig. 2.
Aerosol-spread during chest compression at the simulation model.
Fig. 3.
Aerosol-spread during chest compression with different devices on the patient's face used in the simulation model (left) and in the cadaver model (right).
A face mask on the patient's face deflected the flow of the aerosol to the patient's forehead. The use of an oxygen mask with or without gas flow showed diffuse deflection of the aerosol-spread. These findings are given in Fig. 3.
Using a laryngeal tube or mask without an airway filter led to direct spread into the direction of the tube which means the direction of the professional performing chest compressions. When a supraglottic airway device connected to a filter was used, almost no aerosol-spread was visible (Fig. 4 ).
Fig. 4.
Aerosol-spread during chest compression with a laryngeal tube used in the simulation model (left) and in the cadaver model (right).
Performing intermittent bag-mask ventilation spread aerosol widely distributed. Direct laryngoscopy in contrast to video-assisted laryngoscopy with ongoing chest compressions necessitates physical closeness of the professionals’ face to the aerosol-spread. A clear drape covering video-assisted laryngoscopy was able to reduce aerosol-spread. Fig. 5 shows visualized aerosol-spread during chest compressions in the simulation model using these and other techniques of airway management. For the techniques performed on the cadaver-model similar results were seen with less extensive aerosol spread.
Fig. 5.
Aerosol-spread during chest compression at the simulation model with different techniques of airway management.
Discussion
COVID-19 is a highly contagious disease with a large number of patients require intensive care medicine.2, 5, 8 Critical care is an integral component in patient treatment but contains aerosol-spreading procedures which potentially lead to infection of medical professionals.8 There is a big discussion whether chest compressions generates aerosol. In their Consensus on Science with Treatment Recommendations, the International Liaison Committee on Resuscitation (ILCOR) suggests that chest compressions have the potential to generate aerosol.9 This accords to previous publications and guidelines.1, 2, 3 That seems reasonable since chest compressions generates passive ventilation with small tidal volumes.10 According to a recent systematic review, there is no evidence for or against aerosol production during chest compressions. We do not know if and when data about aerosol generation during chest compressions will be available. Concerning this ongoing lack of information, we focused our investigation about methods to reduce the spread of artificial generated aerosol during chest compression. Our study therefore investigates protection against a potential harm, not against a proven harm.4 In this context our model is designed to generate artificial aerosol which may or may not be generated by chest compression or positive pressure ventilation during CPR. At this juncture there is little data or recommendation for CPR in times of COVID-19. The Resuscitation Council UK recommends using a face mask for oxygen therapy to minimize aerosol-spread during CPR.7 At the moment, there is insufficient data whether the potential risk for healthcare providers during CPR may be caused by droplets or aerosol. Data from chest physiotherapy and non-invasive ventilation suggests the spread of droplets and not aerosol during these procedures.11 Personal protective equipment differs between droplet and airborne precautions. An inserted laryngeal tube with an airway filter may be effective to reduce both, aerosol and droplet spread. In our simulation, applying a face mask leads to deflection of simulated aerosol but seems not to be sufficient. The use of an oxygen mask with or without flow led to diffuse spread of aerosol. Our findings suggest that the early insertion of a laryngeal tube or a laryngeal mask connected to an airway filter during chest compression reduces the aerosol-spread. Since a lot of inpatient cases develop an acute respiratory distress syndrome (ARDS), early airway management and oxygenation are the mainstay of the treatment for acute deterioration or cardiac arrest.12 There is data showing non-inferiority for supraglottic airway devices versus endotracheal intubation in out of hospital cardiac arrest.13, 14 Endotracheal intubation in COVID-19 patients is a high risk procedure and should be performed by specialists with maximum expertise to reduce the risk of infection.1, 3, 7, 15 This procedure may be delayed due to donning of personal protection equipment (PPE) of a specialized team. Furthermore, bag mask ventilation (BMV) also seems to have a high risk for infection and should be avoided.3 We suppose that you need a continuously tight-fitting mask on the patients face to reduce aerosol-spread during BVM, which needs two hands for e.g. a double C-E technique. Especially based on safety precautions, this seems not practical during CPR in our opinion. The early insertion of a supraglottic airway device by any advanced life support provider particularly for staff protection may be an alternative in the setting of cardiac arrest since its quickly insertion from a bigger distance compared to direct laryngoscopy. Video-assisted laryngoscopy covered by a clear drape may reduce aerosol-spread. Performance of airway management – even before starting chest compression – is in contrast with actual guidelines of the European Resuscitation Council.16 Early airway management in cardiac arrest of COVID-19 seems to be reasonable since literature names respiratory failure the main cause of death in these patients.17 Despite using a difficult airway trainer to simulate human upper airway, it remains a low-fidelity simulation. In our simulation model we used continuous air flow on the nebulizer to simulate aerosol-generation. To create realistic conditions at the cadaver model we filled the residual volume with aerosol and did not apply any continuous flow. Because of an ethanol-based fixation detergent, the human cadavers provide more realistic conditions referring to elasticity of the thorax and mechanical properties of the lung. Still the residual capacity of the lungs may be reduced due to partial filling with the ethanol-based agent for fixing the cadavers. Because of this, our cadaver model may underestimate the amount of air exiting the lung during chest compression. Since the amount of dead space might differ between the cadavers, the amount of aerosol expelled by chest compressions might also differ. In patient care, dead space anatomy will also be variable. Both components of our experimental setup showed very similar results, giving us confidence about the validity of the chest compression simulation model. Nevertheless, we do not know how close we can get to realistic conditions using an aerosol-spreading model like this. Citing the ICLOR statement, there remains a knowledge gap about the potential for aerosol generation during CPR.9 As an additional limitation, we do not know whether aerosol or droplet spread during CPR translates to an actual transmission risk of SARS-CoV-2 to the healthcare providers. In spite of that, our simulation model is easy to build and may be a good tool for clinical training. We believe that we basically imitated an aerosol-spread during chest compressions with our experimental setup. For further confirmation we applied this model to human cadaver and saw our first results fundamentally confirmed. Both setups are still models of aerosol-spread, but we generally see comparability to human resuscitation. Resuscitation studies are worthwhile, but difficult to perform. Further investigations as for instance measurements of environmental virus particles may generate more precise answers about contagiosity during CPR.
Conclusion
CPR and airway management are high risk procedures regarding to a potential infection with SARS-CoV-2. With the visualization of aerosol-spread during chest compression by ultraviolet sensitive detergents on an ex situ simulation model and on a cadaver model with we generated a model for better understanding of the safety measures to protect medical professionals.
We state that the early insertion of a supraglottic airway device connected to an airway filter before starting chest compressions may be a good method for both – treating potential hypoxemia and protect the advanced life support providers. Being aware of the limitations of our models and in times of short amounts of PPE, further studies about aerosol-spread during resuscitation are necessary.
Conflict of interest
None declared.
Contributor Information
Matthias Ott, Email: ma.ott@klinikum-stuttgart.de.
Alfio Milazzo, Email: alfio.milazzo@uni-tuebingen.de.
Stefan Liebau, Email: stefan.liebau@uni-tuebingen.de.
Christina Jaki, Email: c.jaki@klinikum-stuttgart.de.
Tobias Schilling, Email: t.schilling@klinikum-stuttgart.de.
Alexander Krohn, Email: a.krohn@klinikum-stuttgart.de.
Johannes Heymer, Email: j.heymer@klinikum-stuttgart.de.
References
- 1.Alhazzani W., Møller M.H., Arabi Y.M. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo M.-Z., Huang Y.-G., Ma W.-H. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin Med Sci J. 2020 doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun-Hei Cheung J., Tin Ho L., Vincent Cheng J., Yin Kwan Cham E., Ngai Lam K. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir. 2020 doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couper K., Taylor-Phillips S., Grove A. COVID-19 in cardiac arrest and infection risk to rescuers: a systematic review. Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 7.Resuscitation Council (UK) 2020. Guidance for the resuscitation of COVID-19 patients in hospital. 2020-03-19. (Accessed 19 March 2020, at https://www.resus.org.uk/_resources/assets/attachment/full/0/36100.pdf) [Google Scholar]
- 8.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 9.On behalf of the ILCOR. Couper K., Taylor-Phillips S., Grove A. International Liaison Committee on Resuscitation (ILCOR); Brussels, Belgium: 2020. COVID-19 infection risk to rescuers from patients in cardiac arrest. Consensus on Science with Treatment Recommendations [Internet] (Accessed 10 April 2020, at http://ilcor.org) [Google Scholar]
- 10.Deakin C.D., O’Neill J.F., Tabor T. Does compression-only cardiopulmonary resuscitation generate adequate passive ventilation during cardiac arrest? Resuscitation. 2007;75:53–59. doi: 10.1016/j.resuscitation.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Simonds A.K., Hanak A., Chatwin M. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess (Rockv) 2010 doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 12.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 13.Benger J.R., Kirby K., Black S. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome the AIRWAYS-2 randomized clinical trial. JAMA. 2018;320:779–791. doi: 10.1001/jama.2018.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H.E., Schmicker R.H., Daya M.R. Effect of a strategy of initial laryngeal tube insertion vs endotracheal intubation on 72-hour survival in adults with out-of-hospital cardiac arrest a randomized clinical trial. JAMA. 2018;320:769–778. doi: 10.1001/jama.2018.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluge S., Janssens U., Welte T., Weber-Carstens S., Marx G., Karagiannidis C. Empfehlungen zur intensivmedizinischen Therapie von Patienten mit COVID-19. Medizinische Klin – Intensivmed Und Notfallmedizin. 2020:7–9. doi: 10.1007/s00063-020-00674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soar J., Nolan J.P., Boettiger B.W. European Resuscitation Council Guidelines for Resuscitation 2015. Section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]