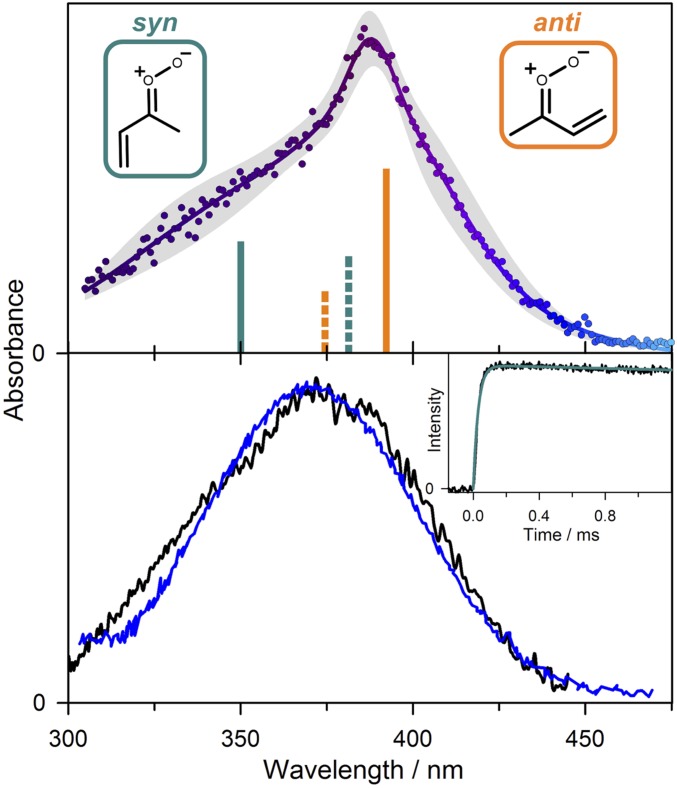
Fig. 1.
(Upper) Electronic spectrum of MVK-oxide recorded under jet-cooled conditions by the UV-Vis induced depletion method from Vansco et al. (31). Data from ref. 31. (Lower) Direct absorption spectra obtained for MVK-oxide at 298 K using the Sandia broadband multipass transient absorption spectrometer (black) and IAMS absorption instrument (blue). Vertical excitation energies and associated oscillator strengths (bars, Upper) computed for the first π*←π transition of MVK-oxide are shown for syn- (cyan) and anti- (orange) conformers; solid and dashed lines further distinguish between trans and cis configurations, respectively, which rapidly interconvert at 298 K. (Lower Inset) Kinetic time trace for MVK-oxide from the Sandia experiment (under 298 K, 10 Torr He; black) integrated between 330 and 367 nm compared with the simulated thermal unimolecular decay for syn-conformers to 2-hydroperoxyl-buta-1,3-diene (see Scheme 2) (cyan) with k(T) = 33 s−1. Thermal rates are computed using ab initio master equation modeling in the high-pressure limit (30). The simulations include an experimental rise time for MVK-oxide appearance of 30 μs from the reaction of the iodoalkenyl radical with O2 (SI Appendix).