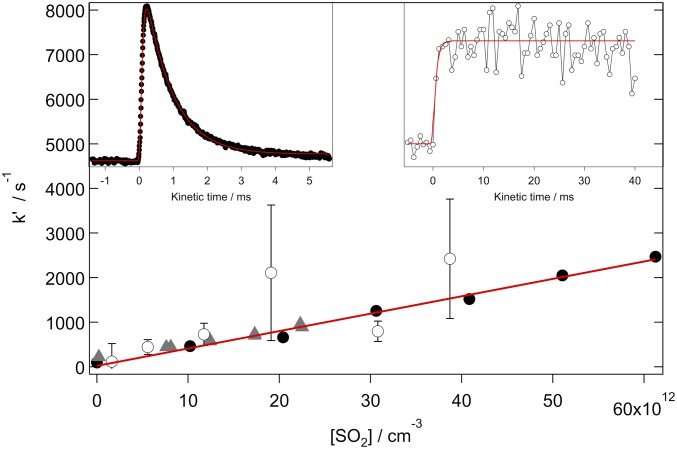
Fig. 3.
First-order rate coefficients for the reaction of syn-MVK-oxide with SO2 as a function of [SO2], obtained from the decay of syn-MVK-oxide using the Sandia absorption experiment (black closed circles; 10 Torr total pressure with He bath gas, 95% confidence limit error bars), the IAMS absorption experiment (gray closed triangles; 4.1 Torr total pressure with O2 bath gas, 340 nm probe wavelength, 95% confidence limit error bars), and SO3 growth from the Sandia MPIMS experiment (open black circles; 10 Torr total pressure with He bath gas, 1σ error bars). Note that error bars for the black closed circles and gray closed triangles are smaller than the symbols. The red line is a linear fit to the Sandia absorption data, weighted by the 95% confidence limit error bars, obtaining a bimolecular rate coefficient of (3.9 ± 0.5) × 10−11 cm3 s−1. (Insets) Typical time-resolved traces from the reaction of syn-MVK-oxide with SO2: syn-MVK-oxide (Sandia absorption, 330 to 367 nm; Top Left Inset) and SO3 (Sandia MPIMS, 13 eV ionization energy; Top Right Inset).