Figure 8.
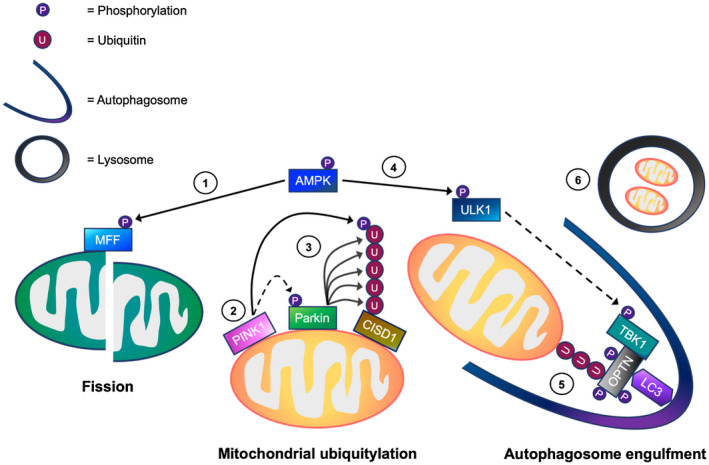
A working model of the mechanisms regulating mitophagy processing in skeletal muscle cells. In response to cellular energy stress, (1) AMP‐activated protein kinase (AMPK) activation initially promotes mitochondrial fission via direct phosphorylation of mitochondrial fission factor (MFF). This allows the separation of healthy and depolarized mitochondria. In depolarized mitochondria, (2) PTEN‐induced kinase 1 (PINK1) accumulates on the outer mitochondrial membrane (OMM), and phosphorylates both ubiquitin (Ub) and Parkin. (3) This is suggested to promote the recruitment of Parkin E3 ubiquitin ligase to the OMM. Parkin then ubiquitylates OMM proteins including CDGSH iron sulfur domain 1 (CISD1), facilitating mitochondrial ubiquitylation. Meanwhile, (4) AMPK activation leads to TBK1 phosphorylation possibly via ULK1, which in turn, is thought to translocate to the mitochondria. (5) The activation of TBK1 is proposed to enhance the binding capacity of autophagy receptors (eg, optineurin, OPTN) to ubiquitylated mitochondria, facilitating autophagosome engulfment. (6) Subsequently, the autophagosome fuses with the lysosome for mitochondrial degradation. → = Signaling thought to occur in skeletal muscle. ⇢ = Assumption based on signaling events in non‐muscle cell lines
