Fig. 2.
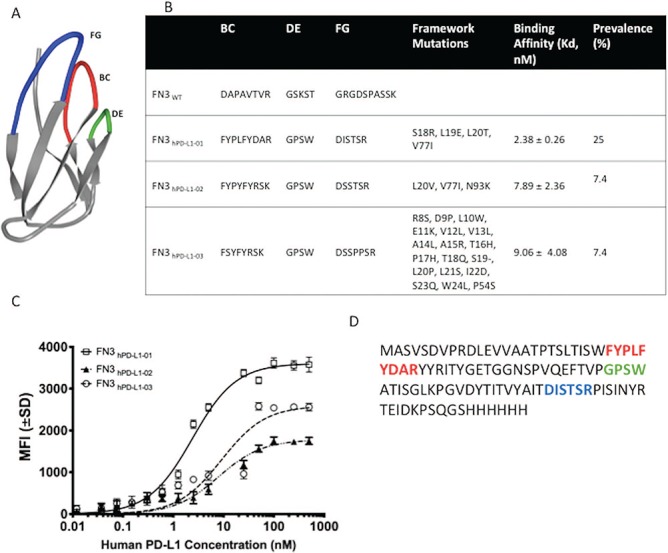
Engineering and development of a high-affinity binder for human PD-L1. (A) NMR structure of wild-type fibronectin domain (PDB ID: 1TTG) showing the engineered loops—BC (top middle), DE (top right) and FG (top left). (B) The diverse sequences of the framework and loops—BC, DE and FG between the three unique clones (FN3hPD-L1-01 to FN3hPD-L1-03) isolated from the mixed binder populations enriched at 60 pM (Fig. 1D) along with their binding affinities (Kd) are shown. (C) Binding dynamics of each yeast clone at different concentrations of purified hPD-L1. Error bars represent S.D. for duplicate samples. (D) Full-length amino acid sequence of the FN3hPD-L1-01 clone within the BC, DE and FG loops, along with the 6x His-tag at the C-terminus. All subsequent experiments were performed using the FN3hPD-L1-01 clone.
