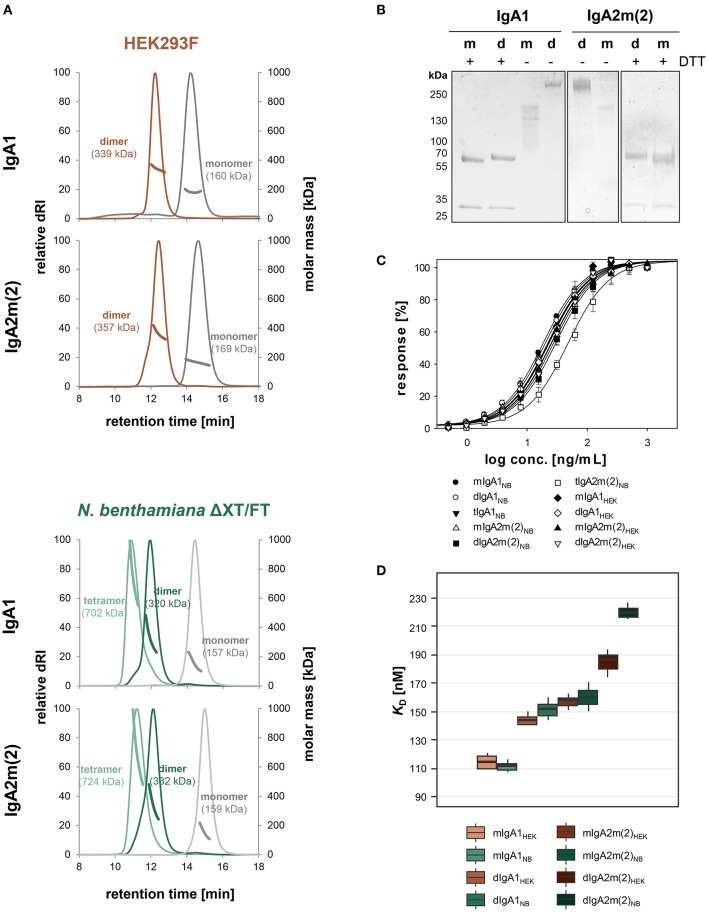
Figure 3.
Biophysical and functional characterization of recombinant monomeric, dimeric and tetrameric IgA. (A) Overlay of normalized SE-HPLC-MALS chromatograms of affinity and gel-filtration purified IgA1 and IgA2m(2) monomers, dimers and tetramers produced in HEK293F cells and N. benthamiana ΔXT/FT plants. (B) SDS-PAGE under reducing (+DTT) and non-reducing conditions of purified monomeric (m) and dimeric (d) IgA1 and IgA2m(2) produced in N. benthamiana ΔXT/FT plants followed by Coomassie Brilliant Blue staining. (C) Binding of the IgA variants to the antigen HER2. The EC50 vales were determined as the mean ± standard deviation from three independent measurements. “m” monomeric, “d” dimeric, “t” tetrameric IgA. (D) Binding affinities of IgA1 and IgA2m(2) monomers and dimers to FcαRI. KD values were obtained by SPR spectroscopy in single-cycle kinetic experiments from three independent measurements. Error bars represent standard deviation.