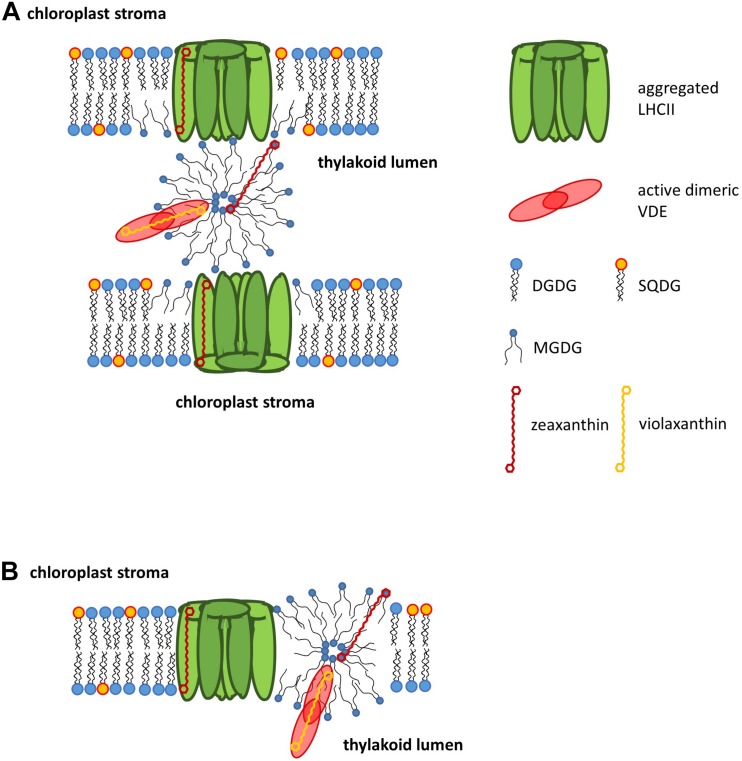
FIGURE 3.
Model for the violaxanthin cycle domain in thylakoid membranes of higher plants. High light illumination leads to a disconnection of the main light-harvesting complex of photosystem II, LHCII from the photosystem II core complex followed by protein aggregation. MGDG molecules, which during low light illumination surround the LHCII, dissociate, segregate and form a non-bilayer lipid phase. This non-bilayer lipid phase may be located outside of the membrane bilayer, i.e., within the thylakoid lumen (A), or within the plane of the thylakoid membrane (B). During high light illumination violaxanthin disconnects from its binding site at the LHCII apoproteins and diffuses into the non-bilayer lipid phase. The non-bilayer lipid phase represents an attraction site for the enzyme violaxanthin de-epoxidase (VDE), which, after its pH-dependent activation and dimerization, binds to the non-bilayer lipid phase. The non-bilayer lipid phase is characterized by a reduced surface tension and thus allows the penetration of the enzyme’s hydrophobic catalytic site into the hydrophobic core of the non-bilayer lipid phase where it gains access to the hydrophobic pigment violaxanthin. After the conversion of violaxanthin to zeaxanthin, zeaxanthin rebinds to the LHCII and participates in photoprotection via NPQ. Since the violaxanthin cycle domain, consisting of LHCII, MGDG, VDE and xanthophyll cycle pigments, is only established during high light illumination of plants, the domain can be described as a transient membrane domain.