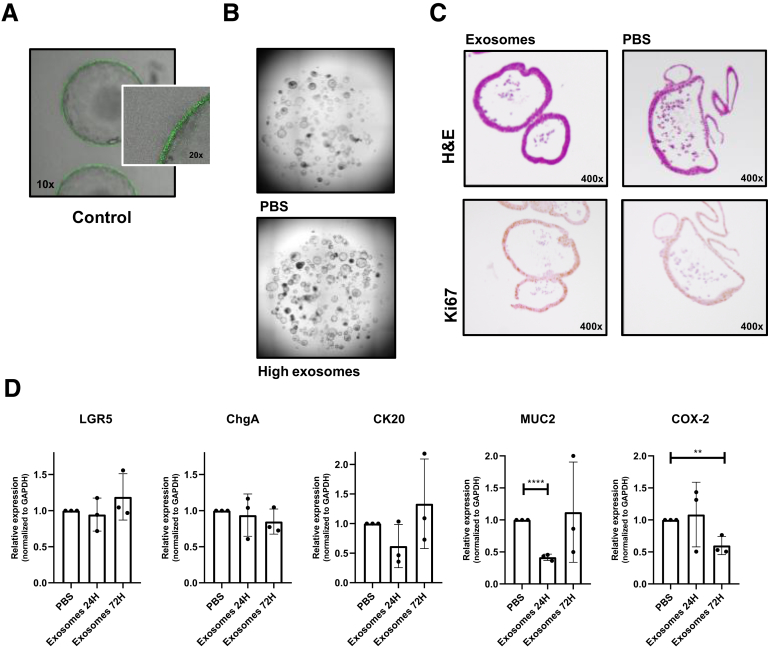
Supplementary Figure 3.
Gene expression and proliferation in MSC exosome–treated organoids. (A) Images of green fluorescent protein-positive colon organoids cultured with a mock control containing vesicle-free fetal calf serum for 1 week. Images are representative of 2 independent experiments. (B) Representative images of colon organoids cultured without and with MSC exosomes for 72 hours. To obtain colon organoids, after killing the mice, the colonic tissue was transferred to 20 mmol/L EDTA (324503; Merck Millipore) in Hanks’ balanced salt solution (14175-053; Gibco) and incubated for 30 minutes at 37°C with repeated vortexing to release the colonic crypts. The crypts were washed in Ad-DF+++, consisting of Advanced Dulbecco's modified Eagle medium/Ham’s F-12 (12634-010; Gibco) supplemented with 1% Glutamax (35050-038; Gibco), 1% penicillin/streptomycin (15140-122; Gibco) and 1% HEPES (15630-056; Gibco). After centrifugation, the colonic crypts were plated in 20 μL Matrigel (growth factor–reduced Matrigel, 356231; Corning, Corning, NY) in 48-well culture plates. After polymerization of the Matrigel, 250 μL of complete growth medium was added, consisting of Ad-DF+++, supplemented with B27 (11530536; Invitrogen, Carlsbad, CA), N-acetylcysteine (A9165-5; Sigma-Aldrich), nicotinamide (n0636; Sigma-Aldrich), A83-01 (2939; Tocris, Bristol, UK), p38 inhibitor (s7067; Sigma-Aldrich), epidermal growth factor (PMG8041; Invitrogen), 20% Noggin CM, 10% R-spondin CM (both kindly provided by the Tytgat Institute, Amsterdam, The Netherlands), and 50% Wnt3a CM (kindly provided by the Hubrecht Institute, Utrecht University, The Netherlands). During the first 4 days, medium was supplemented with Rho-K inhibitor (Y0503; Sigma-Aldrich). Colonic organoids were maintained in a humidified incubator at 37°C containing 5% CO2. (C) H&E and Ki67 staining of colon organoids cultured with exosomes or phosphate-buffered saline (PBS) for 72 hours. Colonic organoids treated with or without exosomes were stained immunohistochemically for the proliferation marker rabbit anti-Ki67 (clone SP6, 16667; Abcam) as described before.1 (D) Relative messenger RNA expression of LGR5, chromogranin A (ChgA), cytokeratin 20 (CK20), cyclo-oxygenase-2 (COX-2), and mucin (MUC2) in dissociated colon organoids co-cultured with PBS or exosomes for 24 and 72 hours. Data represent the means of 3 independent experiments performed in triplicate. The Student t test was used. Messenger RNA was isolated using the nucleospin RNA kit (740955250; Macherey-Nagel, Düren, Germany) after 24 and 72 hours. Complementary DNA was generated using the RevertAid First Strand Complementary DNA Synthesis Kit (ThermoFisher Scientific) according to the manufacturer’s protocol. Quantitative polymerase chain reactions were performed using SYBR green (1708886; Bio-Rad, Hercules, CA) and primers (all Invitrogen) for a stem cell marker (leucine-rich repeat-containing G-protein–coupled receptor 5: forward: TGGTGGCTTTGACCGTGTT; reverse: CGATTACCCCAATTAGCAGCTTT), differentiation markers (mucin 2: forward: CCCAGAGAGTTTGGAGAGCA; reverse: CTCCTCACATGTGGTCTGGT; chromogranin A: forward: GGCCCAGCAGCCGCTGAAGCAGCA; reverse: CTCTGCGGTTGGCGCTGCCCTCCTC; cytokeratin 20: forward: CGCATCTCTGTCTCCAAAGC; reverse: TTCTGCATTGCCAGTTTCCC), and the prostaglandin pathway (cyclo-oxygenase 2: forward: CCGTGCTGCTCTGTCTTAAC; reverse: TTGGGAACCCTTCTTTGTTC). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene: forward: AACTTTGGCATTGTGGAAGG; reverse ACACATTGGGGGTAGGAACA. **P < .01, ****P < .0001.